Figure 5.
Correction of Dmd Gene in Soft 3D Fibrin-Expanded MuSCs Using Adenoviral Vector Delivery of RNA-Guided CRISPR/Cas9 and Donor DNA
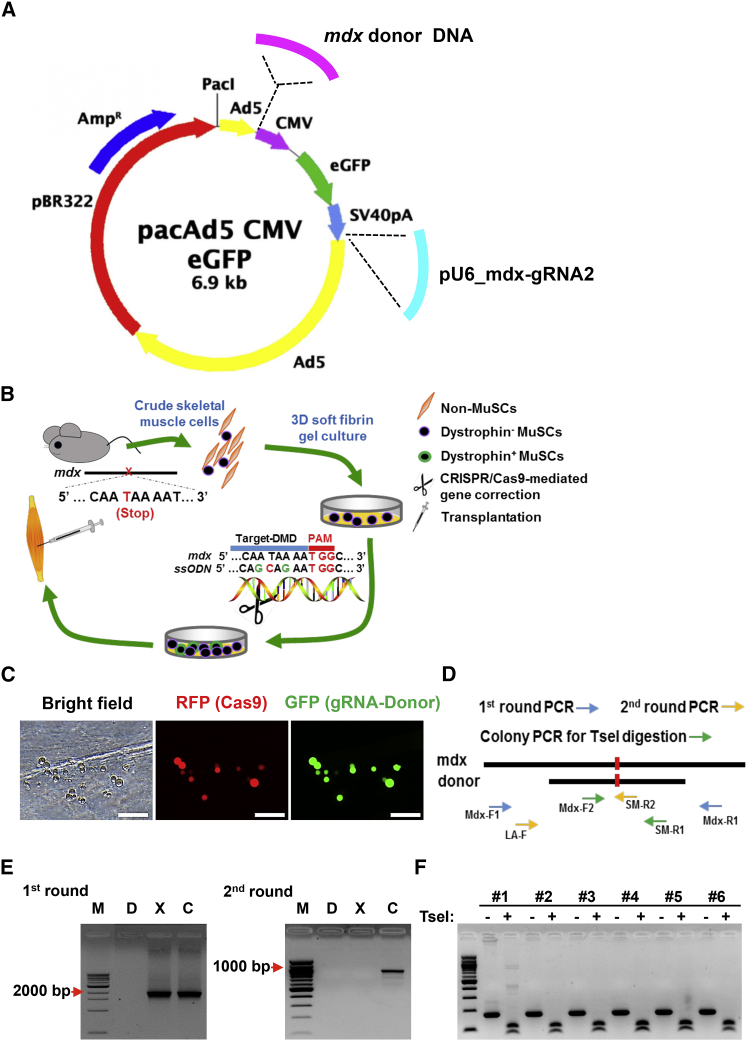
(A) Diagram of adenoviral vectors expressing Cas9 (AdV-Cas9) and harboring gRNA expression cassette and Dmd-specific donor template. The mdx-gRNA2 shown in Figure 2B was used. The donor template was a 1,314-bp DNA fragment with 591- and 722-bp homology arms flanking the mutation site. The silent mutations constituting TseI restriction enzyme site in ssODN were also incorporated in this longer donor template. (B) Scheme of adult MuSC-based gene therapy for DMD in mdx mice. Bulk skeletal muscle cells were isolated from mdx mice and then cultured in soft 3D fibrin gel. When morphologically round MuSCs became evident (3∼4 days), CRISPR/Cas9 and donor DNA complexes were delivered to initiate targeted genome editing for correcting Dmd mutations. Cells were allowed to expand in fibrin gel for 3 more days to propagate Dmd-corrected MuSCs. Expanded MuSCs were then transplanted in mdx mice. (C) Representative images showing adenoviral delivery of CRISPR/Cas9 components into fibrin-expanded cells. Bulk skeletal muscle cells were cultured in soft 3D fibrin gel. When round MuSCs became the dominant cell type in soft 3D fibrin gel, cells were coinfected with adenoviruses expressing CRISPR/Cas9 (red) and carrying gRNA and donor DNA (green). RFP was used to track infection efficiency of adenovirus expressing Cas9, whereas GFP was used to reflect transfection efficiency of donor DNA and gRNA. Scale bar, 100 μm. (D) Schematic diagram for assessing correction of Dmd mutation by adenoviral delivery of CRISPR/Cas9 complexes. To avoid interference of donor DNA as template in PCR, genome DNA was amplified first by a pair of primers (Mdx-F1 and Mdx-R1) residing outside of donor DNA sequence. The resultant PCR products containing both original and mdx-corrected fragments were then purified and subcloned into TOPO-TA cloning vector. After transformation, single bacterial colonies were picked up for allele-specific PCR using primers of LA-F and SM-R2 to screen for the corrected clones. To confirm allele-specific PCR results, colony PCR was further performed using primers of Mdx-F2 and SM-R1, and the resultant product was subjected to TseI digestion. TseI site was designed and introduced in the donor DNA. (E) Genotyping result of HDR-mediated Dmd correction in the fibrin-expanded MuSCs (n = 3 independent experiments). M, 100-bp DNA marker; D, donor DNA plasmids; X, genomic DNA from uncorrected mdx muscle cells; C, genomic DNA from corrected fibrin-expanded mdx muscle cells. Mdx-F1 and Mdx-R1 were used for the first round of PCR, and LA-F and SM-R2 were used for the second round of PCR. (F) TseI digestion confirming HDR-mediated Dmd correction in fibrin gel-expanded MuSCs. DNA fragments (lane C, left panel of D) from first round of PCR were sub-cloned into TOPO-TA cloning vector, followed by colony-PCR with primers of Mdx-F2 and SM-R1. PCR products from individual colonies were directly digested by TseI.