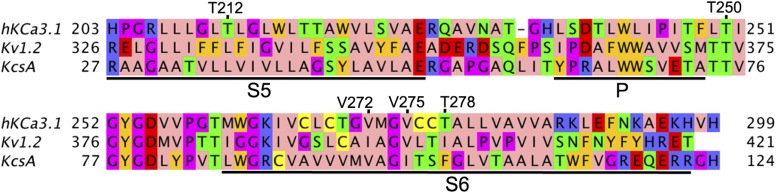
Fig. 1.
Sequence alignment between the KCa3.1, Kv2.1, and KcsA channel pore-forming domains. Transmembrane regions S5 and S6 and the pore P-helix are underlined by black bars and labeled. Positions of residues forming a part of the drug receptor sites discussed in this paper are marked and labeled. Amino acids were colored with the Jalview program (http://www.ebi.ac.uk/∼michele/jalview) (Clamp et al., 2004; Waterhouse et al., 2009) using the Zappo color scheme, where hydrophobic residues (I, L, V, A, and M) are colored pink; aromatic residues (F, W, and Y) are colored orange; positively charged residues (K, R, and H) are colored blue; negatively charged residues (D and E) are colored red; hydrophilic residues (S, T, N, and Q) are colored green; P and G colored magenta; and C is colored yellow.