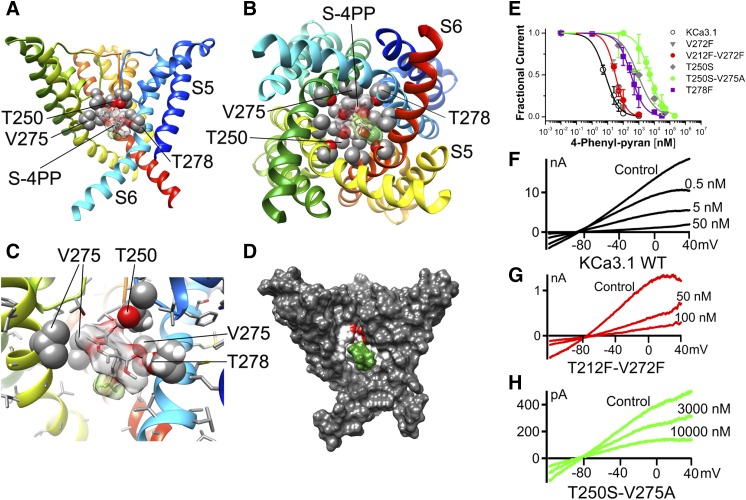
Fig. 8.
(S)-4-Phenyl-pyran interaction with KCa3.1 channel pore. (A) Transmembrane view of the ribbon representation of one of the lowest-energy models of the KCa3.1 channel complex with (S)-4-phenyl-pyran (shown in stick representation with a transparent molecular surface). Only three subunits are shown for clarity and each subunit is colored individually. Side chains of T250, V275, and T278 are shown in space-filling representation and labeled. Transmembrane segments S5 and S6 are labeled for one of the subunits. (B) Intracellular view of the model shown in (A) with all four subunits shown. (C) Close-up of the transmembrane view shown in (A). (D) Surface representation of the transmembrane view of the model shown in (A). Only three subunits are shown for clarity. T250, V275, and T278 side chain atoms are colored light gray (carbon), white (hydrogen), and red (oxygen). S-4-phenyl-pyran is colored green. (E) Concentration-dependent inhibition of wild-type (WT) and mutant KCa3.1 channels by 4-phenyl-pyran. WT KCa3.1 is inhibited by 4-phenyl-pyran with an IC50 value of 7.7 ± 4.5 nM (h = 1.20 ± 0.27; n = 5). While the pore mutants T250S (IC50 = 969.9 ± 242.7 nM; h = 0.61 ± 0.16; n = 3), T250S-V275A (IC50 = 4.45 ± 1.63 µM; h = 1.02 ± 0.36; n = 7), and T278F (IC50 = 321.04 ± 130.82 nM; h = 0.97 ± 0.38; n = 11) decrease the 4-phenyl-pyran block by 125-, 575-, and 40-fold, respectively, neither of the fenestration mutants V272F (IC50 = 30.1 ± 2.4 nM; h = 1.65 ± 0.48; n = 3) or T212F-V272F (IC50 = 30.2± 4.5 nM; h = 1.54 ± 0.25; n = 5) significantly reduce the 4-phenyl-pyran block. Data points are expressed as mean ± S.D.; n = number of independent cells used to construct the concentration-response curves. (F–H) Current traces showing examples of inhibition of WT KCa3.1, T212-V272F, and T250S-T275A currents by 4-phenyl-pyran.