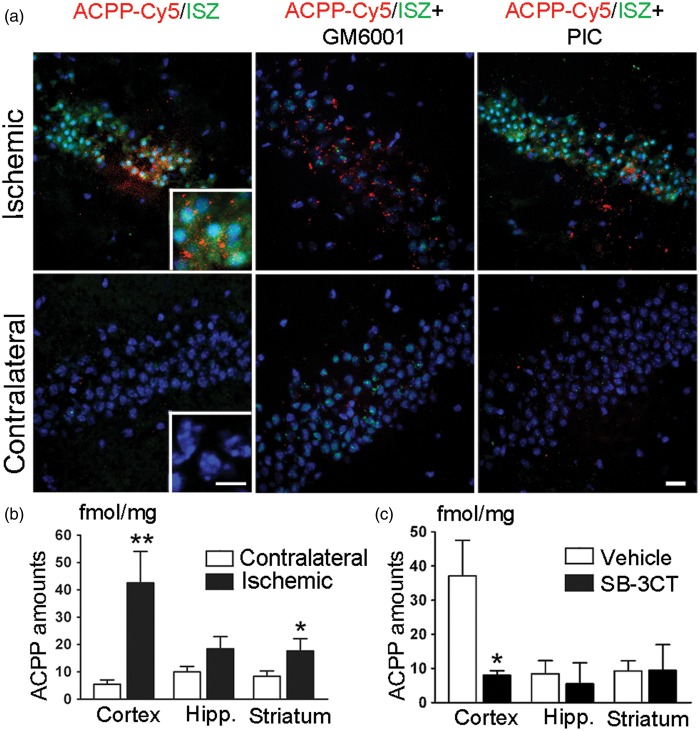
Figure 4.
Detection of gelatinase activity by ACPPs in mouse brain after MCA occlusion. (a) Representative micrographs of ACPP-Cy5 uptake and in situ zymography (ISZ) in the hippocampal CA3 subregion. ACPP-Cy5 (2 nmol) was injected i.v. after 2-h filament-induced MCA occlusion in mice, which were sacrificed 24 h later. ACPP-Cy5 uptake by cells in the brain parenchyma displayed a similar pattern of distribution to increased DQ-gelatin-FITC labeling in the ischemic CA3 region, but not in the contralateral hippocampus. MMP inhibitor GM6001applied ex vivo to brain frozen sections attenuated the ISZ fluorescence of DQ-gelatin-FITC in the ischemic hippocampus, but did not affect uptake of ACPP-Cy5. As a negative control, the non-MMP protease inhibitor cocktail (PIC) did not reduce ISZ fluorescence intensity. Brain sections were counterstained with Hoechst to visualize nuclei (blue). Scale bars, 20 µm, and 10 µm (inset). (b) ACPP-Cy5 uptake was quantified ex vivo in different brain regions 24 h post-ischemia. ACPP-Cy5 uptake was detected at fmol/mg levels and significantly increased in the ischemic cortex (**p < 0.005, n = 8) and striatum (*p < 0.05, n = 10) compared to contralateral control. (c) Differences in ACPP-Cy5 uptake between ischemic and contralateral regions were compared after SB-3CT treatment or vehicle-treated control. ACPP-Cy5 uptake was significantly attenuated by the gelatinase selective inhibitor SB-3CT in the cerebral cortex (*p < 0.05, n = 8 vehicle-treated mice, n = 6 SB-3CT treated mice). Data are expressed as mean ± SEM. Statistics determined by one-tailed Student’s t-test.