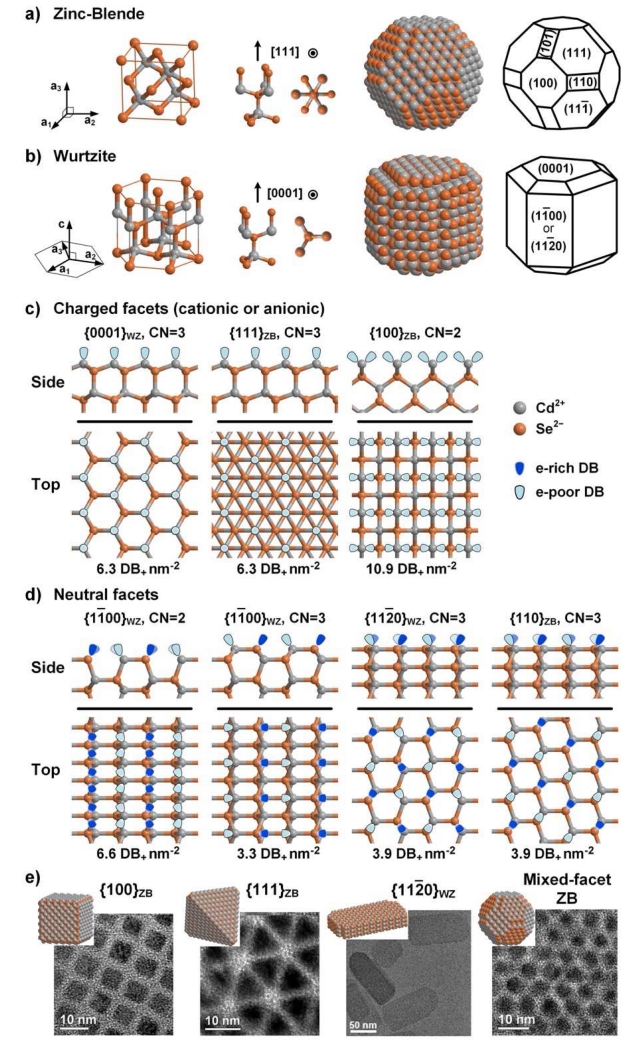
Fig. 2.
Surface facet chemistry of ionic nanocrystals with zinc blende (ZB) or wurtzite (WZ) crystal phases, showing CdSe examples. (a,b) Bonding geometries and structures of ZB and WZ QDs. From left to right: crystal phase unit vectors, unit cell, two views of the internal bonding geometry parallel to, and perpendicular to, the [111]ZB and [0001]WZ direction, and two schematics of nanocrystals indicating atomic locations and lattice facet identifications. Cd2+ ions are grey and Se2− ions are orange. (c,d) Surface facets are depicted from two orientations (side and top views). As shown, they are “bare” without ligands and without reconstruction. Dangling bonds (DBs) are shown indicating whether they are electron-rich on Se2− or electron-poor on Cd2+. (c) Charged facets have either cationic Cd2+-rich surfaces or anionic Se2−-rich surfaces (only cation-rich surfaces are shown). (d) Neutral facets have equal numbers of cations and anions. (e) Transmission electron micrographs of four nanocrystals are shown with different facets exposed, showing QDs with all {100}ZB, all {111}ZB, all {112̄0}WZ, or mixed facets (reproduced from references [27] and [28] with permission).