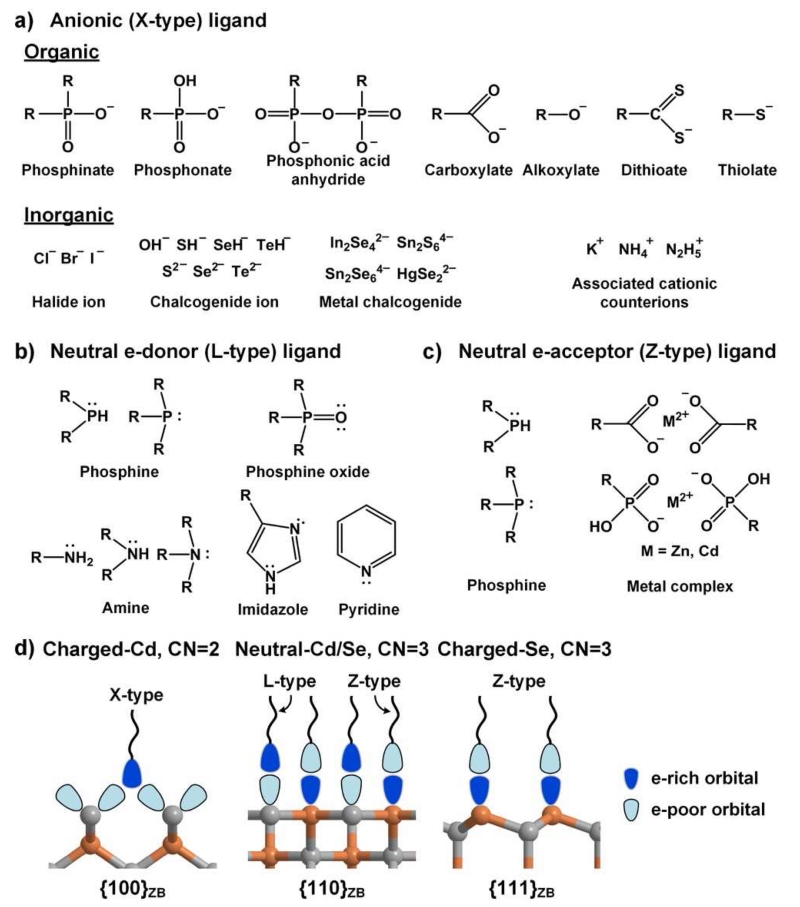
Fig. 3.
Common ligands for colloidal QD facets. (a) Anionic X-type ligands are anions that are either conjugate bases of weak organic acids or inorganic ions, often associated with cationic counterions. (b) Electrostatically neutral, electron-donating L-type ligands have lone pairs of electrons on heteroatoms and/or delocalized π electrons. (c) Neutral electron-accepting Z-type ligands are electron-withdrawing metal cations in complexes with X-type ligands, or L-type ligands that can also function as electron acceptors. (d) The expected preferential orientations of ligands on different types of facets, based on X-ray crystal structures and theoretical atomistic studies, with orbitals shown in shading indicating their degree of filling. X-type ligands are charged and preferentially bind to cationic facets, typically in a bridging conformation between two adjacent surface cations. Electrostatically neutral L-type and Z-type ligands can bind to neutral facets that present both electron-rich and electron-deficient DBs. Z-type ligands bind weakly to anionic surfaces.