Figure 1. Structural models of receptor-catalyzed nucleotide exchange.
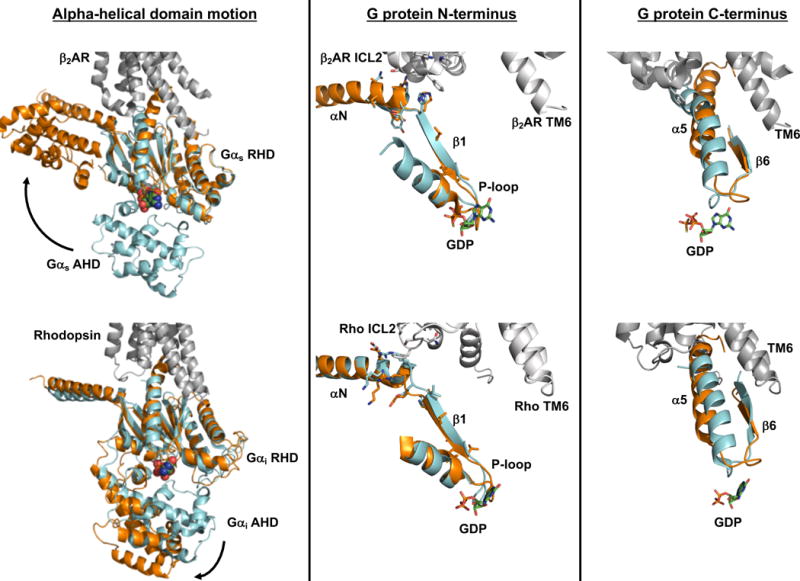
Multiple lines of evidence suggest that a separation of the G protein ras-homology domain (RHD) and alpha-helical domain (AHD) is necessary to exchange bound GDP for GTP. While motion of the alpha helical domain was observed in the crystal structure of β2AR in complex with nucleotide-free Gs heterotrimer (top left) and in a model of rhodopsin in complex with GDP-bound Gi heterotrimer (bottom left), domain separation is likely not sufficient to trigger GDP release from Gα. Rather, binding to an activated receptor stabilizes conformational changes within the G protein that disrupt nucleotide interactions with the RHD. Interaction of intracellular loop 2 (ICL2) of the receptor with the αN helix and αN-β1 junction of the G protein leads to a reorganization of the P-loop that coordinates the β-phosphate of GDP (middle column). Furthermore, the C-terminal α5 helix of Gα undergoes a rotation and translation to occupy its binding site within the hydrophobic core of the receptor that is opened upon outward movement of TM6. Movement of the α5 helix in turn alters the β6-α5 loop that directly contacts GDP (right column). Similar receptor-G protein contacts, and G protein conformational changes, are seen in the structure of β2AR-Gs complex (top panels) and the model of the rhodopsin-Gi complex (bottom panels). In both scenarios, receptor is shown in gray (PDB: 3SN6 for β2AR-Gs; Rho*-Gi is from Alexander et al. [43]). For comparison of receptor-bound and unbound Gαs in the top panels, the RHD of GTPγS-bound Gαs (PDB: 1AZT, cyan) was aligned to the RHD of Gαs in the β2AR complex (PDB: 3SN6, orange). A similar alignment was used in the bottom panels to overlay GDP-bound Gαi (PDB: 1GOT, cyan) with the RHD of receptor-bound Gαi (orange).
