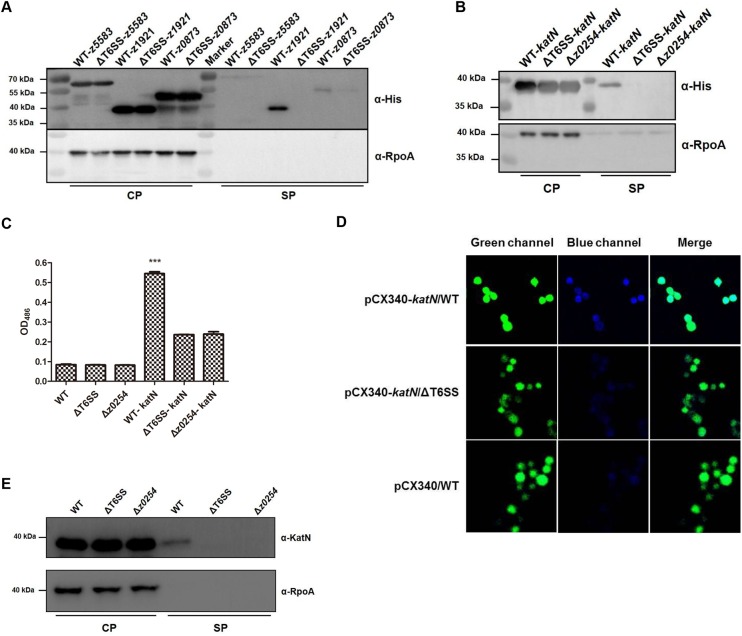
Fig 4. The secretion of KatN is dependent on T6SS in EHEC.
(A) Verification of the T6SS effector candidates by Western blot. The WT and ΔT6SS bearing pQE80-z1921(katN) or z5583 or z0873 with a His-tag sequence fusion at the C-termini were cultured to an OD600 = 1.0 in LB broth at 37°C. The pellet and supernatant fractions of the cultures were analyzed by Western blot using the anti-His tag and anti-RpoA monoclonal antibodies. RpoA was used as an internal control. Three biological repeats were performed. (B) z0254 is required for the secretion of KatN. The WT, Δz0254 and ΔT6SS harboring pQE80-z1921(katN) with a His-tag sequence fusion at the C-terminus were cultured to an OD600 = 1.0 in LB broth at 37°C. The pellet and supernatant fractions of the cultures were analyzed by Western blot using the anti-His tag and anti-RpoA monoclonal antibodies. RpoA was used as an internal control. Three biological repeats were performed. (C) The β-lactamase fusion assay of KatN. The WT, Δz0254 and ΔT6SS bearing pCX340 or pCX-z1921(katN) were cultured to an OD600 = 1.0 in LB broth at 37°C. The β-lactamase activities in the supernatants of the cultures were detected by monitoring optical density change at 486 nm. The β-lactamase activity data (means ± SD) represented the results from duplicate samples of three biological repeats. ***, P<0.001, ANOVA analysis. (D) TEM-1 β-lactamase translocation assay. RAW264.7 cells were infected with EHEC strain EDL933 (WT) or ΔT6SS bearing pCX340 or pCX-katN, and visualized by fluorescence microscopy with excitation at 409 nm or 488 nm after being treated by CCF2-AM. Emission due to CCF2-AM can be viewed as green fluorescence at 520 nm, whereas disruption of CCF2-AM by Bla fusion protein activity results in emission at 447 nm (blue fluorescence). One representative field was shown for each strain, and the assay was conducted at least twice. (E) The secretion of KatN is confirmed by Western blot using the anti-KatN antibody. The WT, Δz0254, Δz0254 bearing pACYC184-z0254 were cultured to an OD600 = 1.0 in LB broth at 37°C. The pellet and supernatant fractions of the cultures were analyzed by Western blot using the anti-KatN and anti-RpoA antibodies. RpoA was used as an internal control. Three biological repeats were performed.