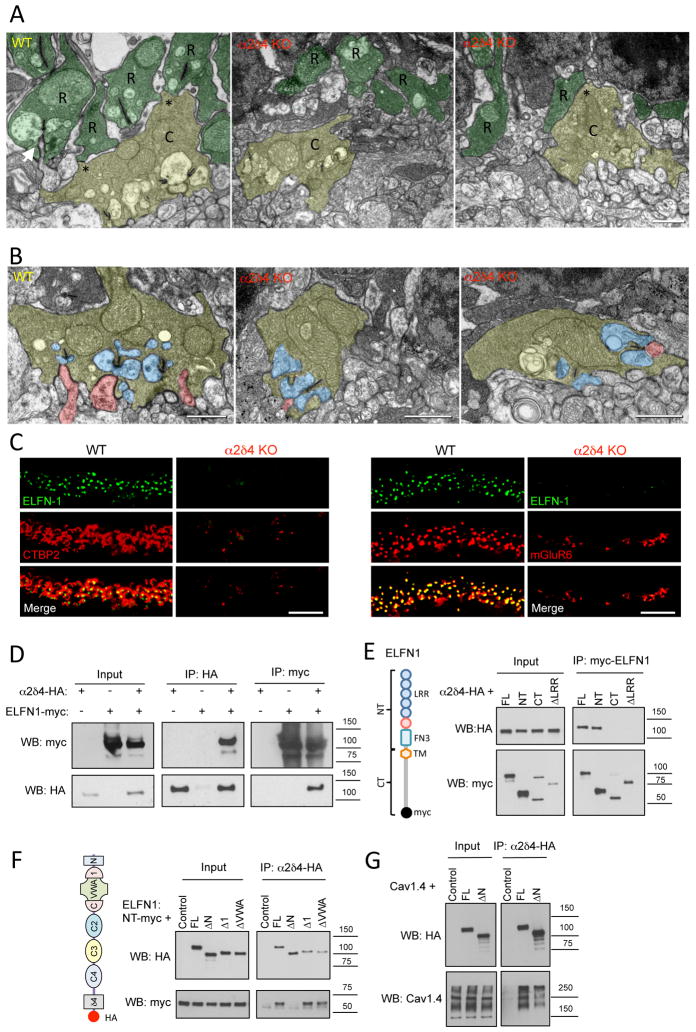
Figure 7. Inactivation of α2δ4 specifically disrupts rod synaptogenesis.
A, Ultrastructural analysis of rod and cone terminals in the OPL of WT and α2δ4 KO retinas. Rod axons and terminals (R) are marked in dark green. Cone pedicles (C) are marked in yellow. Gap junction-like contacts between rod and cone terminals are identified by asterisks. Arrow indicates ON-RBC dendrite invaginating into rod spherule. Scale bar, 500 nm. B, Morphology of cone photoreceptor terminals revealed by electron microscopy. Retinas from two different mice for each genotype were used. Cone terminals (pedicles) are colored in yellow, processes of horizontal cells in blue and ON-BC dendrites in pink. Scale bar, 500nm. C, Complete loss of cell adhesion protein ELFN1 in rod synapses of α2δ4 KO. Scale bar, 10μm. Retinas from three different mice for each genotype were used. D, α2δ4 and ELFN1 co-immunoprecipitate upon co-expression in HEK293 cells. Three independent experiments were performed. E, Site-directed mutagenesis to delineate determinants in ELFN1 responsible for binding to α2δ4. Various structural features present in ELFN1 (diagram) were deleted, and truncated constructs were probed for binding to α2δ4 upon co-expression in HEK293 cells. Three independent experiments were performed. F, Site-directed mutagenesis to delineate determinants in α2δ4 responsible for binding to ELFN1. Various structural features present in α2δ4 (diagram) were deleted and the truncated constructs were probed for binding to ELFN1 upon co-expression in HEK293 cells. Three independent experiments were performed. G, Disrupting N-terminal region of α2δ4 does not prevent its interaction with CaV1.4 as evidenced by co-immunoprecipitation assay performed as in panel F, except that ELFN1 was substituted with CaV1.4. Three independent experiments were performed.