Fig. 1.
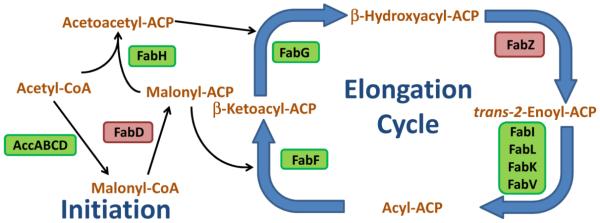
The conserved, core enzymes in bacterial FASII. The first committed step of fatty acid synthesis is the irreversible carboxylation of acetyl-CoA by the acetyl-CoA carboxylase enzyme complex to make malonyl-CoA, the building block of fatty acids. Next, malonyl-CoA is converted into malonyl-ACP by FabD. FabH initiates fatty acid synthesis by catalyzing the Claisen condensation of acetyl-CoA with malonyl-ACP to make acetoacetyl-ACP. From here, the acyl-ACP is elongated 2 carbons per cycle by the elongation enzymes. First, the β-ketoacyl-ACP (including acetoacetyl-ACP) is reduced by FabG to make β-hydroxyacyl-ACP. Next, β-hydroxyacyl-ACP is dehydrated by FabZ to make trans-2-enoyl-ACP. trans-2-Enoyl-ACP is reduced by enoyl-ACP reductase to make acyl-ACP. Acyl-ACP is elongated by the Claisen condensation reaction with a malonyl-ACP by FabF to increase the acyl chain by another 2 carbons and start another round of elongation. Acyl-ACP of sufficient length is used by the acyltransferase system to make phosphatidic acid from glycerol-3-phosphate. Certain bacteria make branched-chain anteiso fatty acids. The FabH of these bacteria use 2-methylbutyryl-CoA instead of acetyl-CoA in the first Claisen condensation reaction. The rate-limiting enzymatic steps that would make good drug targets are in a green box. The equilibrium enzymatic steps that would make poor drug targets are in a red box.