Figure 9.
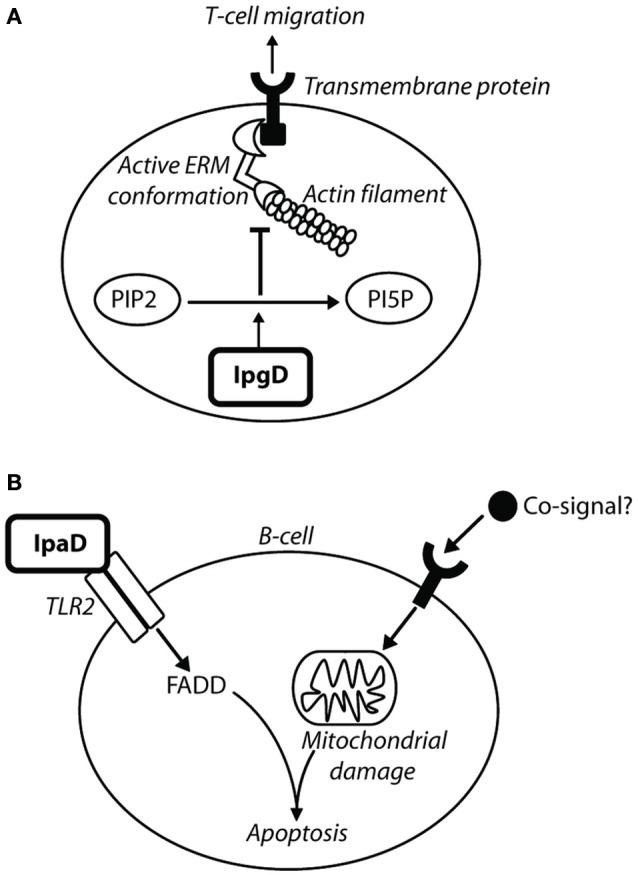
IpgD inhibits T-cell migration and IpaD induces B-cell apoptosis. (A) The PIP2 concentration at the plasma membrane is responsible for the dynamic interchange between active and inactive conformations of ezrin, radixin, and moesin (ERM) proteins (Konradt et al., 2011). ERM proteins are involved in cell-cortex organization of T-cells, and their active conformations localize at the membrane in response to chemokine stimulation to allow T-cell migration toward chemoattractants (Konradt et al., 2011). In the subcapsular sinus of the lymph node (Salgado-Pabón et al., 2013) Shigella can invade T-cells or inject IpgD via the T3SS into T-cells to reduce PtdIns(4,5)P2 concentration. This prevents cell polarisation induced by active ERM proteins and T-cell migration to sites of infection (Konradt et al., 2011). (B) Bacterial co-signals increase pro-apoptotic proteins, induce loss of mitochondrial membrane potential (MMP), and also upregulate tlr2 mRNA, leading to increased TLR2 expression on the surface of the cell. IpaD signaling via the TLR2-1 heterodimer leads to up-regulation of FAS-associated death domain (FADD) protein, which ultimately induces B-cell apoptosis (Nothelfer et al., 2014).
