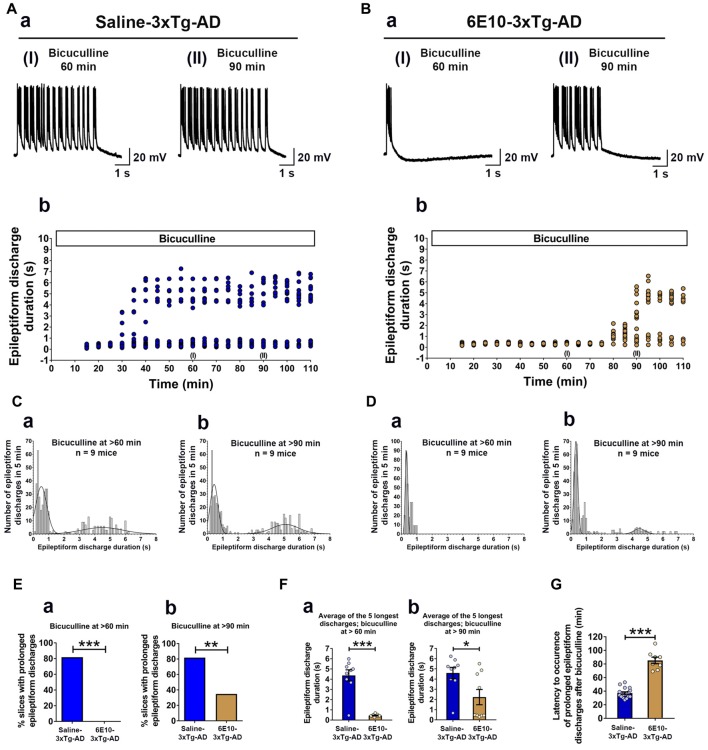
Figure 6.
Suppression of ictal-like activity by passive immunization with anti-human APP/Aβ antibody, 6E10, in hippocampal slices from 3-week-old 3xTg-AD mice. (A) CA3 intracellular recordings from a Saline-3xTg-AD slice after bicuculline application (50 μM). Continuous perfusion with bicuculline induced prolonged synchronized epileptiform discharges (>1.5 s) in Saline-3xTg-AD slice which are depicted at 60 min (AaI) and 90 min (AaII) after bicuculline application. Membrane potential at the beginning of recordings: −73 mV (60 min) and −75 mV (90 min). (Ab) Plot of the duration of epileptiform discharges recorded in the cell shown in (Aa). (B) CA3 intracellular recordings from a 6E10–3xTg-AD slice revealed only short epileptiform discharges 60 min after bicuculline application (BaI). Fully developed prolonged epileptiform discharges were not observed until 90 min after bicuculline (BaII). Membrane potential at the beginning of recordings: −76 mV (60 min) and −74 mV (90 min). (Bb) Plot of the duration of epileptiform discharges recorded in the cell shown in (Ba). (C) Frequency histograms of the duration of all synchronized discharges during a 5-min period after 60 min (Ca) and 90 min (Cb) of bicuculline perfusion in Saline-3xTg-AD mice (n = 22 slices from n = 9 mice). At 60 min after bicuculline, a two-peak distribution showed a group of short epileptiform discharges with average duration of 0.560 ± 0.015 s and a group of prolonged epileptiform discharges of 4.339 ± 0.086 s (second-order Gaussian fit; r = 0.79). Also, at 90 min after bicuculline, a two-peak distribution showed a group of short epileptiform discharges with average duration of 0.553 ± 0.018 s and a group of prolonged epileptiform discharges of 4.951 ± 0.079 s (second-order Gaussian fit; r = 0.83). (D) Frequency histograms of the duration of all epileptiform discharges during a 5-min period after 60 min (Da) and 90 min (Db) of bicuculline perfusion in 6E10–3xTg-AD mice (n = 23 slices from n = 9 mice). At the 60-min time point, all epileptiform discharges were normally distributed with an average duration of 0.475 ± 0.011 s (first-order Gaussian fit; r = 0.91). No prolonged epileptiform discharge was observed in Saline-3xTg-AD mice at 60 min after bicuculline application. However, some of the slices (8 out of 23, 34.8%) exhibited long epileptiform discharges 90 min after bicuculline. At 90 min after bicuculline, a two-peak distribution showed a group of short epileptiform discharges with average duration of 0.466 ± 0.016 s and a group of prolonged epileptiform discharges of 4.195 ± 0.168 s (second-order Gaussian fit; r = 0.88). (E) The overall incidence of prolonged epileptiform discharges both 60 min (Ea) and 90 min (Eb) after bicuculline application was significantly reduced by passive immunization with 6E10 (p < 0.001 and p = 0.0023, respectively; Fischer’s exact test). (F) Passive immunization with 6E10 significantly reduced the average durations of the five longest synchronized epileptiform discharges recorded during 5 min intervals both 60 min (Fa) and 90 min (Fb) after bicuculline (p < 0.001 and p = 0.021, respectively; Student’s t-test). (G) The latency to occurrence of prolonged epileptiform discharges was significantly higher in 6E10–3xTg-AD mice (85.3 ± 4.8 min) as compared to Saline-3xTg-AD mice (37.2 ± 1.7 min; p < 0.001; Student’s t-test). *p < 0.05, **p < 0.01, ***p < 0.001.