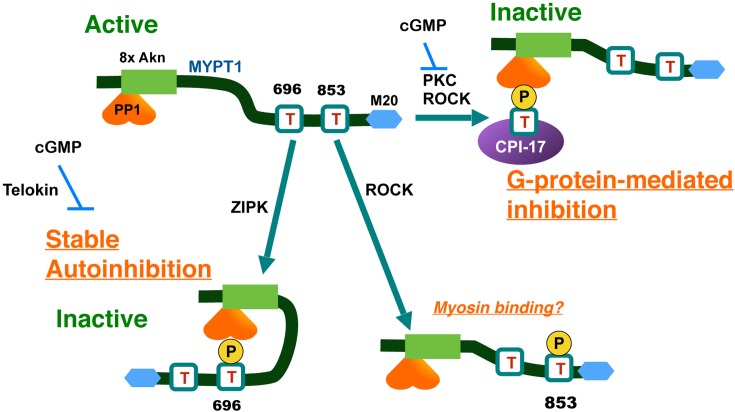
Fig. 1.
The molecular basis of the regulation of MLCP through phosphorylation of MYPT1 and CPI-17. Phosphorylation of MYPT1 at Thr696 spontaneously occurs and causes autoinhibition of MLCP by direct contact to the active site. Thr853 phosphorylation is elevated in response to ROCK activation, but it has no little effects on MLCP activity. In response to GPCR stimulation, PKC and ROCK phosphorylate CPI-17 at Thr38, and the phospho-Thr38 directly docks at the active site of MLCP and inhibits the activity. MYPT1, myosin phosphatase target subunit-1; M20, an accessory subunit of MLCP; CPI-17, PKC-activated PP1 inhibitor protein with Mr of 17 kDa; 8xAkn, 8 ankyrin repeats; PP1, type-1 Ser/Thr phosphatase catalytic subunit.