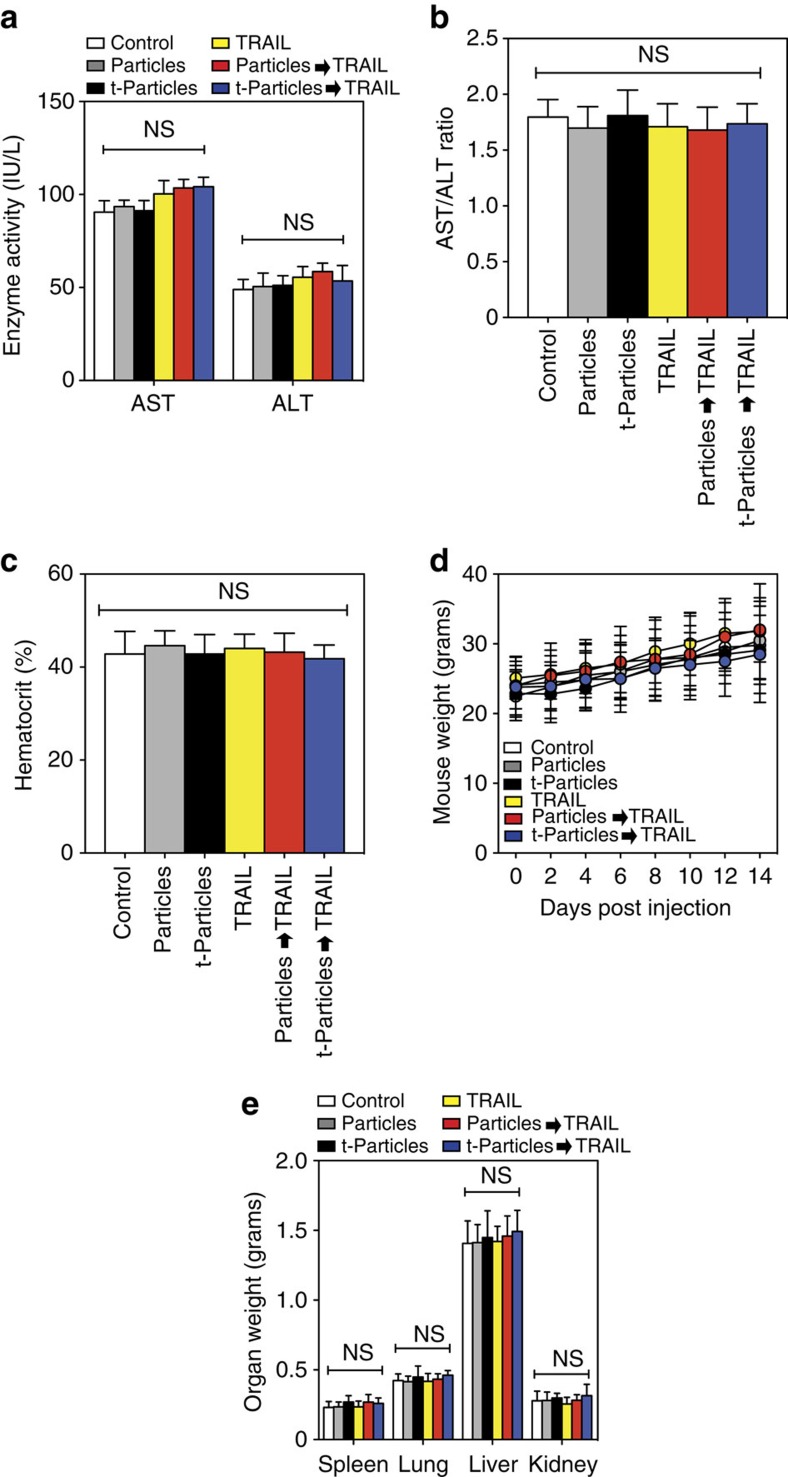
Figure 6. Delivery of nontargeted particles (Particles) and EpCAM-targeted PLGA particles (t-Particles) followed by TRAIL therapeutic does not affect non-target cells and tissues in vivo.
(a) Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) liver enzymes in mice from controls and different treatment groups at the end of the 2-week study. (b) AST/ALT ratio in serum of mice from controls and different treatment groups at the end of the 2-week study. AST/ALT >2 indicates liver toxicity. (c) Haematocrit levels of mice from controls and different treatment groups. Blood was drawn before killing of mice. (d) Weight of mice 0–2 weeks post injection of TRAIL and particle-functionalized tumour cells. (e) Weight of excised organs post-mortem. Data are reported as mean±s.e. Different treatment groups were compared for statistical significance using a one-way analysis of variance (ANOVA) for multiple comparisons. NS, not significant. N=5 mice for all treatments.