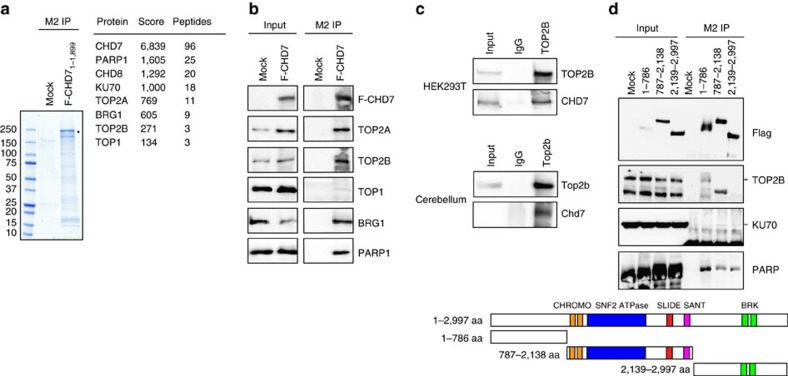
Figure 5. Chd7 interacts with Top2b.
(a) Coomassie-blue staining of an SDS gel shows immunoprecipitation using M2 beads in CHD7 KO HEK293T cells overexpressing a Flag-tagged truncated CHD71–1,899 (highlighted with asterisk). Cells transfected with an empty vector served as mock control. Numbers on the left side indicate the molecular weight. A list of proteins identified by mass spectrometry, peptides of which were exclusively present in CHD7-immunoprecipitation but not mock control, is shown in the right panel. Scores and numbers of unique peptides identified for each protein are shown. (b) Immunoblotting of CHD7-interacting proteins precipitated with M2 beads in nuclear extracts from HEK293T cells overexpressing a Flag-tagged full-length CHD7 (F-CHD7) or mock-transfected cells. Forty per cent of IP and 2.5% of the input are shown. (c) Co-immunoprecipitation assay shows the interaction between endogenous Chd7 and Top2b in HEK293T and adult (6-week-old) mouse cerebella. Cell lysates were immunoprecipitated with an antibody against Top2b, and co-precipitated Chd7 was visualized by western blots. Five per cent of input and 100% of IP are shown. Note that endogenous Chd7 in adult cerebella was only detected by western blot after enrichment by immunoprecipitation. (d) The N-terminal part of CHD7 interacts with TOP2B. Cell lysates from HEK293T cells transfected with a series of constructs encoding Flag-tagged truncated CHD7 proteins were precipitated with M2 beads, and precipitated proteins were visualized by western blots. Fifty per cent of IP and 4% of the input are shown. Bands corresponding to TOP2B and KU70 according to their molecular weight are pointed with lines. The schemes illustrate the domain structure of full-length and truncated CHD7 protein used in the experiments.