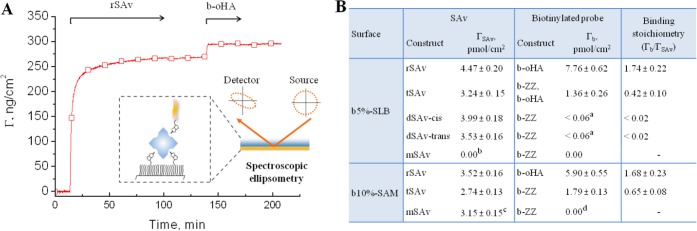
Figure 6.
SAv binding and residual valency on densely biotinylated surfaces, quantified by SE. (A) Example of binding curves obtained by SE, here for rSAv and b-oHA on a b5%-SLB. The inset illustrates the SE setup. (B) Table of surface densities of SAv, ΓSAv, and biotinylated reporter probes (b-ZZ or b-oHA), Γb, and the mean residual valency, Γb/ΓSAv. Values are presented as mean ± error, where the latter is the sum of the reproducibility error (4%; averaged from 4 independent sets of SE measurements with 2 to 4 samples in each set, cf. column 3 in Table S1) and the detection limit of the SE (1 ng/cm2). aBinding was below the detection limit of SE; bmSAv removed biotinylated lipids from b-SLB (cf. Figure 4B); cMeasured before buffer rinsing, as binding was not stable (cf. Figure 4A); db-ZZ accelerated displacement of mSAv from the surface. Conditions: SAv adsorption time = 90 min, biotinylated probe adsorption time = 15–60 min. All values were determined after adsorption and buffer rinsing once the SE response was stabilized, except for mSAv on SAMs, where the equilibrium bound amount before rinsing is given (as rinsing provokes mSAv detachment, Figure 4A).