Table 2. Oxidation of Different Cyclohexane Derivatives.
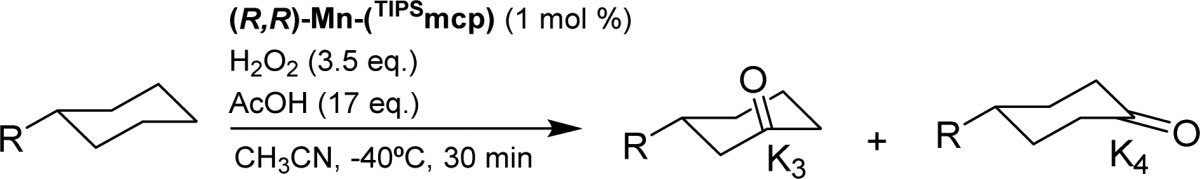
| entry | R | conv (%)a | yield (%)a K3(K4) | K3/K4b | Ee (K3) (%) |
|---|---|---|---|---|---|
| 1 | -t-Bu (S1) | 77 | 53 (19) | 1.4 | 44 |
| 2 | -OPiv (S2) | 80 | 41 (10) | 2.0 | 54 |
| 3 | -Si(Me)3 (S3) | 95 | 42 (13) | 1.6 | 23 |
| 4c | -CO2CH3 (S4) | 88 | 47 (20) | 1.2 | 11 |
| 5 | -CO2H (S5) | 45 | 20 (11) | 0.9 | 9e |
| 6c | -COCH3 (S6) | 95 | 47 (19) | 1.2 | 8 |
| 7 | -NHCOCH3 (S7) | 93 | 74 (3) | 12 | (+)63f |
| 8c | -NHCOCH3 (S7) | 94 | 75 (3) | 12 | (−)78 |
| 9 | -NHCOtBu (S8) | >99 | 90 (1) | 45 | (+)76 |
| 10d | -NHCOtBu (S8) | >99 | 90 (1) | 45 | (−)76 |
| 11c | -NHCOtBu (S8) | >99 | 90 (1) | 45 | (−)85 |
Conversions and yields determined from crude reaction mixtures by GC or 1H NMR.
Normalized ratio.
(S,S)-Mn-(TIPSecp) (2 mol %).
(S,S)-Mn-(TIPSmcp).
Ee’s determined after esterification of isolated products.
Absolute configuration was determined on the basis of the crystal structure of the product obtained from S10 (see Table 4 and Supporting Information). Ee’s determined by GC with chiral stationary phase.
