Table 4. Impact of the Structure of the Acyl Moiety in Regio- and Enantioselective C–H Oxidation of N-Cyclohexyl Amides with (S,S)-Mn(TIPSecp) as Catalyst.
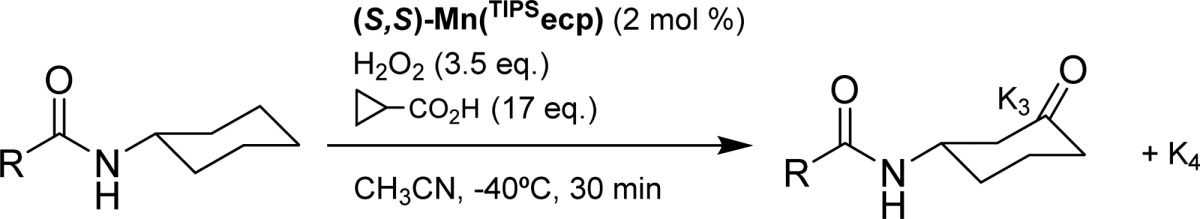
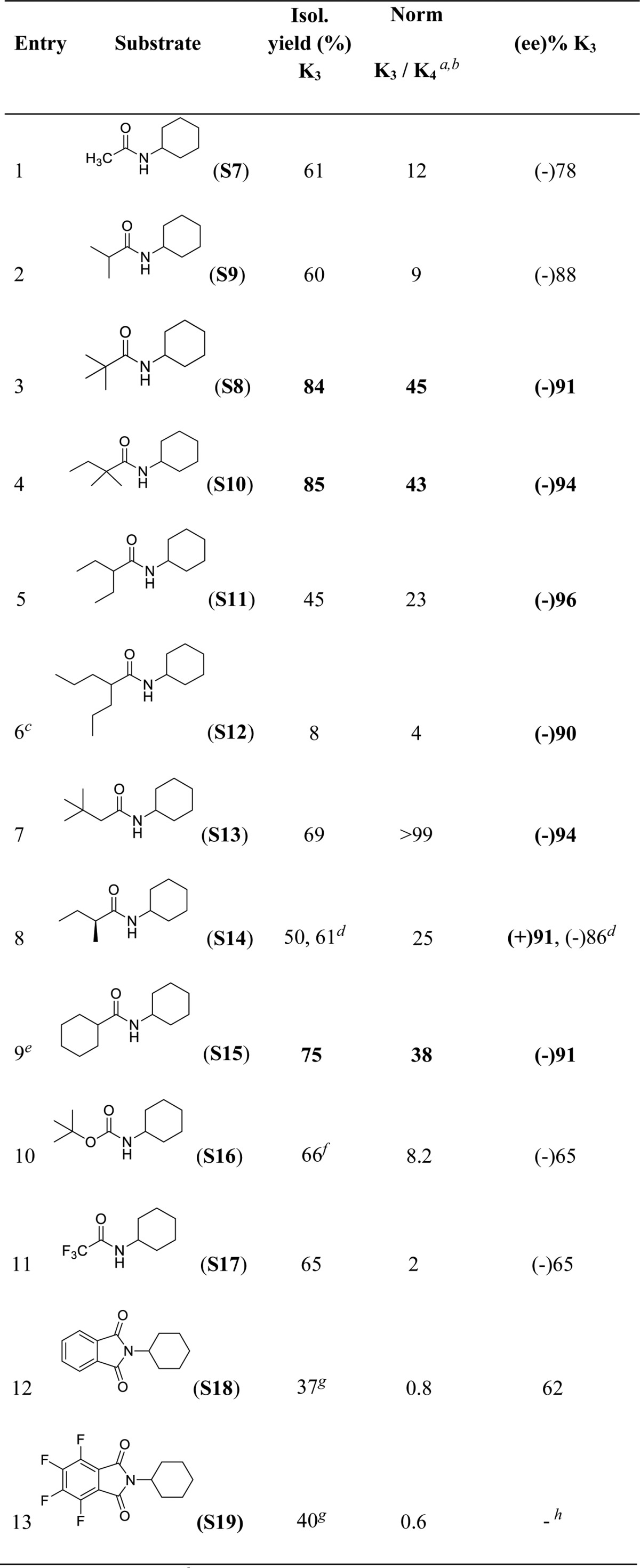
Normalized ratio.
K4 yields determined by GC.
Recovered starting material = 78%.
(R,R)-Mn(TIPSecp).
Acetic acid (17 equiv) instead of cyclopropanecarboxylic acid.
Yield determined by GC.
Products isolated as mixture of (K3 + K4).
Ee not determined. Ee’s determined by chiral GC and HPLC.
