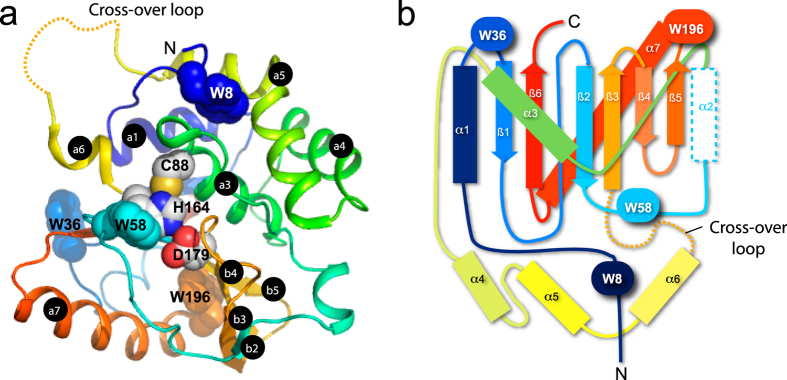
Figure 1. Three-dimensional structure of UCH-L5N240 and its knotted folding topology.
(A) Cartoon representation of the crystal structure of UCH-L5N240 in complex with ubiquitin (PDB ID: 4UEL). UCH-L5N240 is colour-ramped from blue to red from the N- to C-termini and ubiquitin is coloured grey with its C-terminus shown in grey sticks. The side-chain atoms of the catalytic residues are shown in spheres with carbon, nitrogen, oxygen and sulphur atoms shown in white, blue, red and yellow, respectively, and their identities indicated accordingly. The tryptophan side-chains are shown in semi-transparent spheres. The cross-over loop was ill-defined due to its flexibility and is indicated in dashed gold line. (B) Topological representation of UCH-L5N240 illustrating the distribution of the tryptophan residues. The colouring scheme is the same as in (A). α-helices and β-strands are shown in rectangular and arrows, respectively.