Abstract
Background
The hypothalamic-pituitary-adrenal stress axis plays a crucial role in community-acquired pneumonia (CAP), with high cortisol being associated with disease severity and corticosteroid treatment resulting in earlier time to recovery. Our aim in the present study was to compare different glucocorticoid hormones, including cortisol, 11-deoxycortisol, cortisone, and corticosterone, regarding their association with short- and long-term adverse outcomes in a well-defined CAP cohort.
Methods
We prospectively followed 285 patients with CAP from a previous Swiss multicenter trial for a median of 6.1 years and measured different admission glucocorticoid serum levels by liquid chromatography coupled with tandem mass spectrometry. We used adjusted Cox regression models to investigate associations between admission hormone levels and all-cause mortality at different time points.
Results
Mortality was 5.3% after 30 days and increased to 47.3% after 6 years. High admission cortisol was associated with adverse outcome after 30 days (adjusted OR 3.85, 95% CI 1.10–13.49, p = 0.035). In the long term (i.e.,), however, high admission cortisol was associated with better survival (adjusted HR after 3 years 0.53, 95% CI 0.32–0.89, p = 0.017; adjusted HR after 6 years 0.57, 95% CI 0.36–0.90, p = 0.015). Compared with 11-deoxycortisol, cortisone, and corticosterone, cortisol showed the highest association with mortality.
Conclusions
Among different glucocorticoid hormones, cortisol showed the highest association with mortality in CAP. Whereas a more pronounced glucocorticoid stress response on hospital admission was associated with higher short-term adverse outcome, long-term outcome was favorable in these patients. These data should support the correct interpretation of glucocorticoid blood data.
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-017-1656-7) contains supplementary material, which is available to authorized users.
Keywords: Community-acquired pneumonia, Glucocorticoid hormones, Cortisol, 11-Deoxycortisol, Cortisone, Corticosterone, Mortality/outcome prediction, Disease severity, Pneumonia severity index
Background
Activation of the hypothalamic-pituitary-adrenal (HPA) axis and stimulation of the central noradrenergic stress system by cytokines and other mediators are major pathophysiological adaptations in response to infection and inflammation [1–4]. Appropriate activation of the HPA axis during illness is crucial for survival and mirrors the stress level. The effects of cortisol include protection against excessive inflammatory reaction, acute supply of energy, and improvement in hemodynamic status [5, 6].
Endogenous adrenocortical function is of prognostic value in critical illness and sepsis [7–14], of which community-acquired pneumonia (CAP) is a major source [15–17]. Morbidity in CAP is still high also, owing to higher risk for cardiovascular events [18, 19]. Researchers in multiple studies have investigated the adrenal steroid metabolism in relation to CAP-associated outcomes [14, 20–25]. In observational trials, researchers have reported increased admission cortisol levels to be independent predictors of disease severity and short-term mortality in patients across the spectrum of mild to severe CAP [20–24]. Interestingly, the predictive accuracy of free cortisol was not superior to that of total cortisol, independent of serum albumin levels [20]. The prognostic accuracy of cortisol levels in short-term mortality prediction was equal to that of the pneumonia severity index (PSI), but better than the CURB-65 score (confusion of new onset, blood urea nitrogen >7 mmol/L, respiratory rate ≥30 breaths per minute, systolic blood pressure <90 mmHg or diastolic blood pressure ≤60, and age ≥65 years) and routinely measured laboratory parameters such as C-reactive protein (CRP), procalcitonin (PCT), or white blood cell count [20, 21, 23]. In addition, in severe CAP, baseline serum cortisol levels were shown to be independent predictors of mortality with superior predictive ability compared with stimulated cortisol with corticotropin or Δ-cortisol [24]. Despite evidence regarding short-term outcomes, less is known about the predictive potential of glucocorticoid levels in regard to long-term mortality. Aside from that, most studies have been focused on cortisol only, and data regarding other human glucocorticoids, such as 11-deoxycortisol, cortisone, and corticosterone, are largely lacking. Identification of patients at risk for adverse outcome after an index hospitalization for CAP may optimize in-hospital and post-acute care strategies to improve survival. A better description of metabolite signatures in CAP may improve pathophysiological understanding and provide new targets for a more personalized therapy. In the present study, our aim was to study different compounds of the glucocorticoid pathway, including cortisol, 11-deoxycortisol, cortisone, and corticosterone, regarding their association with short- and long-term adverse outcomes in a well-defined CAP cohort.
Methods
Study design and setting
This is a secondary analysis of data from a prospective, randomized, controlled multicenter trial performed at six Swiss secondary or tertiary care centers between October 2006 and March 2008 [26]. A detailed study protocol of the initial trial has been published elsewhere [27]. Briefly, from a total of 1825 potential patients, 925 subjects with CAP were included. The aim of the initial trial was to assess the efficacy and safety of a PCT-guided antibiotic therapy compared with standard guidelines without using PCT data [28–30] in patients with CAP and other lower respiratory tract infections [31]. The study protocol was approved by the ethics committee of the University of Basel as well as by all local ethics committees, and written informed consent was provided by all participants for the initial trial, including agreement to use their data anonymized in secondary analyses.
Selection and assessment of participants
Inclusion criteria were age >18 years and final diagnosis of CAP with an infiltrate on a chest x-ray [31]. Exclusion criteria were language restriction or dementia precluding patients from providing written informed consent, presence of a terminal condition, and intravenous drug abuse. For reasons of the initial antibiotic stewardship trial, patients were excluded for severe immunosuppression or for long-term antibiotic therapy on admission independent of the current infection (e.g., in case of endocarditis or chronic infections), whereas a requirement of corticosteroids or short-term antibiotic pretreatment was allowed. All patients included in the analysis were inpatients.
Patient assessment included clinical and biochemical evaluation upon admission to the emergency department (ED) and throughout the period of hospitalization. The standardized baseline characteristics comprised medical history, comorbidities (identified either through patient self-report or medical chart review), vital signs including fever (≥38.5 °C), laboratory values, chest x-ray, and medication. To assess disease severity, the PSI [32] and the CURB-65 score [29]—validated risk assessment tools to categorize patients with CAP into different risk classes—were calculated upon ED admission. Confusion was also assessed as part of these scores. Discharge decision concerning patients enrolled in the study was left completely to the treating physicians without interference of the study team. For the present study, 285 patients with a final diagnosis of CAP and available serum blood specimens [27, 31] were included.
Analysis of blood biomarker
Within the initial trial, blood samples from each patient were collected and frozen upon ED admission for later measurement of different biomarkers. Cortisol, 11-deoxycortisol, cortisone, and corticosterone levels were measured in admission serum blood samples of all 285 included patients with CAP.
After internal validation studies, we determined concentrations of selected hormones using a commercially available kit (MassChrom Steroids; Chromsystems, Munich, Germany). The analysis was performed using the UltiMate 3000 ultra-high-performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific, San Jose, CA, USA) coupled to an AB Sciex 5500 quadrupole mass spectrometer (AB Sciex, Darmstadt, Germany). The Turbo V ion source (AB Sciex) was operated in positive electrospray ionization mode. The targeted screening method employed the multiple reaction monitoring mode of operation using two transitions for each analysis sample. Prior to injection into the UHPLC system, serum samples were subjected to a complex process of reversed phase 96-well solid-phase extraction, purification, and concentration as described in the MassChrom Steroids user’s manual. Quantification of selected metabolites was achieved by reference to appropriate internal standards. Concentrations of all analyzed metabolites were reported in nanomoles per liter.
Main outcome measurements
The primary endpoint of this study was defined as 6-year all-cause mortality. As secondary endpoints, we reported mortality at days 30, 60, 90, 180, 240, and 300, as well as at 1 year, 2 years, and 3 years. Additional secondary endpoints were 30-day adverse outcome (including all-cause mortality and/or admission to the intensive care unit [ICU]) and disease severity defined by the PSI on admission. The decision for ICU admission was left to the treating physicians.
To validate outcomes, we performed blinded, structured telephone interviews at 30, 180, and 540 days after enrollment, as well as after a median of 6.1 years (IQR 5.6–6.5) [33, 34]. In cases where patients or their family members could not be contacted, the treating general practitioner was contacted.
Statistical analyses
Statistical analyses were conducted using STATA 12.1 software (StataCorp, College Station, TX, USA). p Values <0.05 indicated statistical significance. Description of the study population was performed with descriptive statistics, including median with IQR to express continuous variables and frequency (percent) for categorical variables. The Wilcoxon rank-sum test was used for two-group comparison, whereas frequency comparison was done by chi-square test. All analyses were performed in the overall population.
Correlation analyses of glucocorticoid levels with inflammatory markers were calculated by Spearman’s rank correlation. For multigroup comparisons, the Kruskal-Wallis test was performed. Univariate and multivariate Cox regression models were performed to investigate associations between glucocorticoid levels and all-cause mortality at different time points; these associations were reported as HR with 95% CI. Before being entered into regression models, blood biomarker levels were log-transformed with a base of 10 due to a skewed distribution; after logarithmic transformation, the data approximated a normal distribution. Because of this transformation, HRs and ORs correspond to a tenfold increase in hormone levels. Kaplan-Meier curves were used to illustrate mortality based on glucocorticoid quartiles (highest versus lower three). To assess the association of glucocorticoid levels with other short-term adverse outcomes, we used univariate and multivariate logistic regression models, reported as OR with 95% CI. Significance levels were calculated by chi-square (Wald) test.
Models were adjusted for age, sex, and comorbidities (coronary heart disease, cerebrovascular insult, chronic kidney disease, neoplastic disease). The multivariate adjustment was predefined on the basis of factors known to be associated with mortality (age and comorbidities). We also included sex in the model because of expected differences in adrenal hormone levels based on gender. Initial randomization was not significantly associated with study endpoints and was thus not further considered for the statistical analysis.
Results
Patient population
Among a total of 285 patients with CAP, 135 (47.3%) died during the 6-year follow-up period. The median age of the entire cohort was 71 years, and 60.4% of patients were male. There was a high burden of comorbidities, with 20.7% (n = 59) of patients having underlying coronary heart disease, 15.4% (n = 44) having congestive heart failure, 23.5% (n = 67) having chronic renal failure, 19.3% (n = 55) having diabetes mellitus, and 13.3% (n = 38) having neoplastic disease. We had blood levels available for cortisol in 227 patients (80%), for 11-deoxycortisol in 249 patients (87%), for cortisone in 283 patients (99%), and for corticosterone in 285 patients (100%). Additional baseline characteristics of the entire cohort, stratified by the primary endpoint as well as by 30-day adverse outcome, are shown in Table 1 and Additional file 1: Table S1, respectively.
Table 1.
Baseline characteristics overall and stratified by 6-year vital status in community-acquired pneumonia
| 6-Year vital status | ||||
|---|---|---|---|---|
| Characteristics | Entire cohort (n = 285) | Survivors (n = 150) | Nonsurvivors (n = 135) | p Value |
| Demographic characteristics | ||||
| Age, years | 71 [57–81] | 64 [45–75] | 79 [70–84] | <0.001 |
| Male sex | 172 (60.4%) | 80 (53.3%) | 92 (68.1%) | 0.011 |
| CAP characteristics | ||||
| PSI class | ||||
| I | 32 (11.2%) | 30 (20.0%) | 2 (1.5%) | <0.001 |
| II | 55 (19.3%) | 44 (29.3%) | 11 (8.1%) | <0.001 |
| III | 52 (18.2%) | 29 (19.3%) | 23 (17.0%) | 0.62 |
| IV | 104 (36.5%) | 38 (25.3%) | 66 (48.9%) | <0.001 |
| V | 42 (14.7%) | 9 (6.0%) | 33 (24.4%) | <0.001 |
| CURB-65 score | ||||
| 0 | 63 (22.1%) | 51 (34.0%) | 12 (8.9%) | <0.001 |
| I | 67 (23.5%) | 41 (27.3%) | 26 (19.3%) | 0.11 |
| II | 82 (28.8%) | 33 (22.0%) | 49 (36.3%) | 0.008 |
| III | 57 (20.0%) | 21 (14.0%) | 36 (26.7%) | 0.008 |
| IV/V | 16 (5.6%) | 4 (2.7%) | 12 (8.9%) | 0.023 |
| Comorbiditiesa | ||||
| Coronary heart disease | 59 (20.7%) | 16 (10.7%) | 43 (31.9%) | <0.001 |
| Congestive heart failure | 44 (15.4%) | 7 (4.7%) | 37 (27.4%) | <0.001 |
| Cerebrovascular insult | 28 (9.8%) | 9 (6.0%) | 19 (14.1%) | 0.022 |
| PAOD | 17 (6.0%) | 7 (4.7%) | 10 (7.4%) | 0.33 |
| Chronic renal failure | 67 (23.5%) | 19 (12.7%) | 48 (35.6%) | <0.001 |
| Diabetes mellitus | 55 (19.3%) | 22 (14.7%) | 33 (24.4%) | 0.037 |
| Neoplastic disease | 38 (13.3%) | 12 (8.0%) | 26 (19.3%) | 0.005 |
| Clinical history | ||||
| Fever | 185 (65.1%) | 113 (75.3%) | 72 (53.7%) | <0.001 |
| Chills | 87 (34.0%) | 58 (42.0%) | 29 (24.6%) | 0.003 |
| Glucocorticoid pretreatment | 22 (7.9%) | 5 (3.4%) | 17 (12.9%) | 0.003 |
| Clinical findings | ||||
| Confusion | 20 (7.9%) | 4 (2.9%) | 16 (13.7%) | 0.002 |
| Body temperature, °C | 38 [37.2–38.8] | 38.2 [37.4–39] | 37.8 [37–38.8] | 0.085 |
| Breath rate, breaths/minute | 20 [16–25] | 20 [16–24] | 24 [18–28] | 0.002 |
| Heart rate, beats/minute | 94 [82–105] | 92.5 [83.5–108] | 95 [80–104] | 0.43 |
| SBP, mmHg | 130 [117–148] | 130 [120–148] | 130 [110–149] | 0.17 |
| Arterial pH | 7.46 [7.42–7.49] | 7.46 [7.43–7.50] | 7.45 [7.41–7.49] | 0.013 |
| SIRS criteria | 188 (66.0%) | 93 (62.0%) | 95 (70.4%) | 0.14 |
| Outcome parameters | ||||
| ICU admission | 21 (7.4%) | 9 (6.0%) | 12 (8.9%) | 0.35 |
| Mechanical ventilation | 7 (2.5%) | 2 (1.3%) | 5 (3.7%) | 0.20 |
| Septic shock | 6 (2.1%) | 1 (0.7%) | 5 (3.7%) | 0.075 |
| Length of stay, days | 8 [5–12] | 7 [4–10] | 9 [6–13] | <0.001 |
| Admission laboratory findings | ||||
| CRP, mg/L | 132 [65–252] | 147 [91–265] | 111 [55–249] | 0.037 |
| PCT, μg/L | 0.48 [0.16–3.20] | 0.64 [0.16–3.72] | 0.43 [0.16–1.97] | 0.37 |
| Cortisol, nmol/L | 402 [203.8–723.2] | 431 [189.1–759.2] | 399 [213.1–710.3] | 0.46 |
| 11-Deoxycortisol, nmol/L | 0.6 [0.17–2.23] | 0.4 [0.16–2.13] | 0.8 [0.18–2.25] | 0.49 |
| Cortisone, nmol/L | 32.5 [18.42–46.72] | 33.2 [19.28–48.49] | 32.4 [17.68–44.41] | 0.29 |
| Corticosterone, nmol/L | 8.7 [2.81–25.02] | 8.9 [2.73–28.67] | 8.4 [3.13–22.95] | 0.85 |
Abbreviations: CAP Community-acquired pneumonia, CRP C-reactive protein, CURB-65 Confusion of new onset, blood urea nitrogen >7 mmol/L, respiratory rate ≥30 breaths per minute, systolic blood pressure <90 mmHg or diastolic blood pressure ≤60, and age ≥65 years, PAOD Peripheral arterial occlusive disease, PCT Procalcitonin, PSI Pneumonia severity index, SBP Systolic blood pressure, SIRS Systemic inflammatory response syndrome, ICU Intensive care unit
Data are presented as median [IQR] or number (percent); p < 0.05 is considered statistically significant. Bold values indicate statistical significance
aComorbidities were identified on the basis of medical records or patient report
Importantly, compared with the study population of the initial trial [26], including 925 patients with CAP with an all-cause mortality of 45% over a follow-up period of 6 years, the present analyzed cohort displayed a similar pattern of baseline characteristics [34]. Mortality in this cohort was 5.3% after 30 days and increased to 20.7% and 47.3% after 3 years and 6 years, respectively.
Time-dependent association between glucocorticoid levels and adverse outcome
Table 2 illustrates associations between admission serum glucocorticoid levels and all-cause mortality in a time-dependent manner. In the short term, all glucocorticoid hormones had HRs >1 and thus tended to be associated with higher mortality risk, but these associations changed over time. We found high initial cortisol and cortisone levels to be significantly associated with lower risk for 6-year mortality with HRs of 0.63 (95% CI 0.41–0.99; p = 0.045) and 0.60 (95% CI 0.38–0.95; p = 0.030), respectively. For 3-year mortality, results were similar with HRs of 0.54 (95% CI 0.32–0.90; p = 0.018) and 0.56 (95% CI 0.33–0.94; p = 0.028) for cortisol and cortisone, respectively. For cortisol, these associations remained robust after adjustment for age, sex, and comorbidities. These results are also presented in Fig. 1, where a stepwise decrease in HRs with longer observation time for cortisol and cortisone, and to a lesser extent also for 11-deoxycortisol and corticosterone, can be observed. We further show Kaplan-Meier curves for 6-year survival stratified by glucocorticoid-level quartiles (Fig. 2). The highest quartile of admission cortisol and cortisone levels was favorably associated with long-term survival.
Table 2.
Association of admission glucocorticoid levels with short- and long-term all-cause mortality in community-acquired pneumonia
| Entire cohort (N = 285) | All-cause mortality time point | |||||
|---|---|---|---|---|---|---|
| 30 days | 3 years | 6 years | ||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Cortisol | ||||||
| Cox regression analyses | ||||||
| Univariate model | 2.47 (95% CI 0.50–12.18) | 0.268 | 0.54 (95% CI 0.32–0.9) | 0.018 | 0.63 (95% CI 0.41–0.99) | 0.045 |
| Multivariate modela | 1.93 (95% CI 0.51–9.02) | 0.405 | 0.53 (95% CI 0.32–0.89) | 0.017 | 0.57 (95% CI 0.36–0.90) | 0.015 |
| 11-Deoxycortisol | ||||||
| Cox regression analyses | ||||||
| Univariate model | 1.37 (95% CI 0.75–2.53) | 0.309 | 0.95 (95% CI 0.76–1.18) | 0.631 | 1.04 (95% CI 0.86–1.24) | 0.710 |
| Multivariate modela | 1.26 (95% CI 0.65–2.44) | 0.486 | 0.84 (95% CI 0.67–1.05) | 0.126 | 0.91 (95% CI 0.75–1.1) | 0.336 |
| Cortisone | ||||||
| Cox regression analyses | ||||||
| Univariate model | 1.37 (95% CI 0.31–6.02) | 0.677 | 0.56 (95% CI 0.33–0.94) | 0.028 | 0.6 (95% CI 0.38–0.95) | 0.030 |
| Multivariate modela | 2.55 (95% CI 0.38–17.30) | 0.337 | 0.71 (95% CI 0.4–1.25) | 0.237 | 0.76 (95% CI 0.46–1.24) | 0.272 |
| Corticosterone | ||||||
| Cox regression analyses | ||||||
| Univariate model | 1.33 (95% CI 0.63–2.81) | 0.457 | 0.77 (95% CI 0.57–1.03) | 0.082 | 0.85 (95% CI 0.66–1.09) | 0.199 |
| Multivariate modela | 1.21 (95% CI 0.55–2.65) | 0.637 | 0.76 (95% CI 0.57–1.02) | 0.067 | 0.81 (95% CI 0.63–1.03) | 0.084 |
Data for univariate and multivariate Cox regression models are presented as HR (95% CI), p value; p < 0.05 is considered statistically significant. Bold values indicate statistical significance. All hormone levels were log-transformed, and thus the HR corresponds to a tenfold increase in these levels
aMultivariate model is adjusted for age, sex, and comorbidities (coronary artery disease, cerebrovascular disease, chronic kidney disease, neoplastic disease)
Fig. 1.
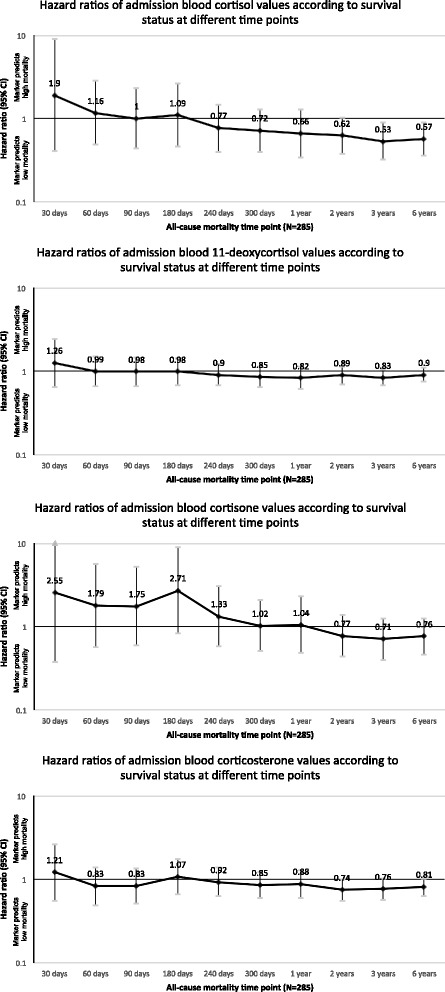
Association of admission glucocorticoid levels with all-cause mortality at different time points in CAP. Data for multivariate Cox regression models are presented as HR (95% CI). HRs >1 reflect a positive association between glucocorticoid levels and all-cause mortality. Multivariate model is adjusted for age, sex, and comorbidities (coronary artery disease, cerebrovascular disease, chronic kidney disease, neoplastic disease). CAP Community-acquired pneumonia
Fig. 2.
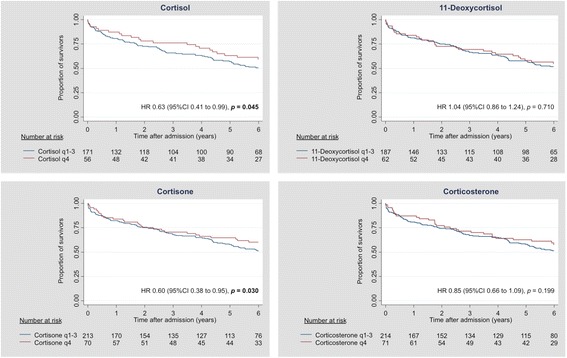
Kaplan-Meier 6-year survival estimate according to admission glucocorticoid levels in CAP: fourth versus first-to-third quartiles. CAP Community-acquired pneumonia
Also, high initial levels of cortisol were independently associated with higher risk for short-term adverse outcome (defined as risk for mortality and/or ICU admission) with an adjusted OR of 3.85 (95% CI 1.10–13.49; p = 0.035) (Table 3).
Table 3.
Association of admission glucocorticoid levels with short-term adverse outcome in community-acquired pneumonia
| Entire cohort (N = 285) | Adverse outcome at 30 days (death and/or ICU admission) | |
|---|---|---|
| OR (95% CI) | p Value | |
| Cortisol | ||
| Logistic regression analyses | ||
| Univariate model | 4.16 (95% CI 1.22–14.12) | 0.022 |
| Multivariate modela | 3.85 (95% CI 1.10–13.49) | 0.035 |
| 11-Deoxycortisol | ||
| Logistic regression analyses | ||
| Univariate model | 1.33 (95% CI 0.84–2.11) | 0.216 |
| Multivariate modela | 1.30 (95% CI 0.80–2.14) | 0.293 |
| Cortisone | ||
| Logistic regression analyses | ||
| Univariate model | 1.89 (95% CI 0.59–1.03) | 0.280 |
| Multivariate modela | 3.25 (95% CI 0.84–12.68) | 0.089 |
| Corticosterone | ||
| Logistic regression analyses | ||
| Univariate model | 1.41 (95% CI 0.81–2.47) | 0.225 |
| Multivariate modela | 1.44 (95% CI 0.79–2.62) | 0.234 |
ICU, Intensive care unit
Data for univariate and multivariate logistic regression models are presented as OR (95% CI), p value; p < 0.05 is considered statistically significant. Bold values indicate statistical significance. All hormone levels were log-transformed, and thus the OR corresponds to a tenfold increase in levels
aMultivariate model is adjusted for age, sex, and comorbidities (coronary artery disease, cerebrovascular disease, chronic kidney disease, neoplastic disease)
Association between glucocorticoid levels and severity of CAP
Multigroup comparison using the Kruskal-Wallis test showed a gradual increase in initial 11-deoxycortisol levels with increasing CAP severity as assessed by PSI classes (p = 0.001) (illustrated in Fig. 3). Contrarily, there was no similar increase in levels of cortisol, cortisone, and corticosterone with PSI. However, for cortisol, there was a trend of increasing hormone levels in more severely ill patients.
Fig. 3.
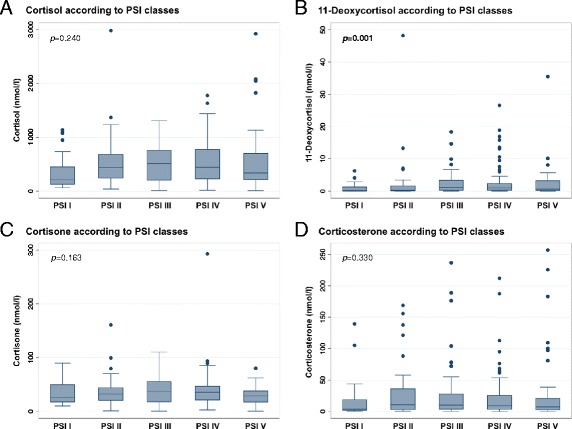
Glucocorticoid levels in patients with various severity classes of CAP. Data represent median and IQR, with scatter plots presenting all values. P values are determined by Kruskal-Wallis test and considered statistically significant at p < 0.05. Bold values indicate statistical significance. CAP Community-acquired pneumonia, PSI Pneumonia severity index
Correlation between glucocorticoid levels and inflammatory markers
As presented in Fig. 4, we found a correlation between admission cortisol levels and high peak CRP (r = +0.22; p = 0.001) and initial PCT values (r = +0.31; p < 0.001). Cortisone levels were also positively correlated with CRP levels (r = +0.14; p = 0.023), whereas 11-deoxycortisol and corticosterone were significantly correlated with PCT levels (r = +0.30 and +0.20, respectively; p < 0.001 for both).
Fig. 4.
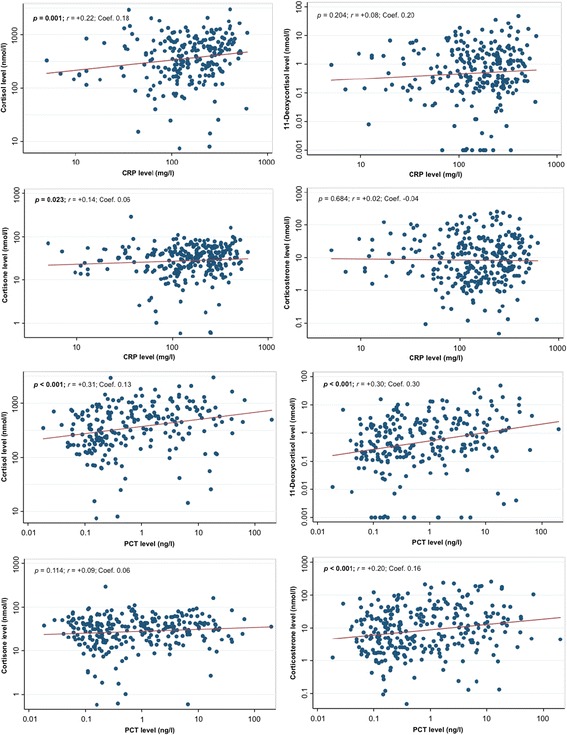
Correlation of admission glucocorticoid levels with inflammatory markers in CAP. Data are presented with scatterplots showing all values (blue), overlaid by linear fit lines (red). Correlation analyses were performed by Spearman’s rank correlation (r; p value). We used multivariate linear regression models to calculate regression coefficients (Coef.). p < 0.05 is considered statistically significant. Bold values indicate statistical significance. We used admission glucocorticoid and PCT levels and high peak CRP levels. CAP Community-acquired pneumonia, CRP C-reactive protein, PCT Procalcitonin. *Multivariate model is adjusted for age, sex, and comorbidities (coronary artery disease, cerebrovascular disease, chronic kidney disease, neoplastic disease)
Discussion
The key findings of our present study of the prognostic value of different glucocorticoid hormones over a follow-up period of 6 years in patients with CAP are threefold. First, among different glucocorticoid hormones, cortisol showed the highest association with outcome in the short and long term. Second, the association of different glucocorticoid metabolites with adverse outcome inversely changed over time; whereas high levels of cortisol on initial hospital presentation were independently associated with short-term adverse outcome, a more pronounced initial glucocorticoid stress response was associated with favorable long-term outcome. Third, admission levels of 11-deoxycortisol showed the best correlation with disease severity.
The HPA stress axis plays an important role in patients with CAP, with cortisol being associated with short-term outcome [20–24] and corticosteroid treatment that might result in earlier time to recovery [35]. The release of cortisol in acute illness is essential for improvement in hemodynamic status, acute supply of energy, and protection against excessive inflammatory reaction [5, 6]. Importantly, the acute stress response involves activation of the glucocorticoid pathway and the adrenergic system, resulting in tachycardia, increased myocardial oxygen consumption, and probably enhanced platelet aggregation, which might translate into acute myocardial infarction, thus potentially raising short-term mortality [18]. Owing to its positive correlation with the degree of illness-induced stress [36], cortisol has been presumed to be a prognostic marker mirroring characteristics of acute critical disease and disease progression. Considering cortisol as the strongest inhibitor of inflammation, a higher level of proinflammatory cytokines in acute illness could lead to a more pronounced increase in cortisol [1–3, 37]. On the basis of several clinical observational studies [20–24], cortisol levels have lately emerged as a useful prognostic tool regarding short-term mortality. The present analysis of a large, well-characterized cohort provides novel insights into the role of the glucocorticoid pathway in regard to adverse outcome in hospitalized patients with mild to severe CAP.
Regarding short-term outcome, our findings are in line with previous data derived from clinical observational studies suggesting that high levels of cortisol ae associated with mortality in CAP [20–24]. In our study, high admission cortisol levels were independently associated with short-term adverse outcome, including the combined endpoint mortality/ICU admission. Association of cortisol levels with 30-day all-cause mortality alone showed a similar trend; likely due to the small event number, the results did not reach statistical significance.
Interestingly, a more pronounced initial glucocorticoid host response was beneficial for long-term survival. For cortisol, this effect remained robust after adjustment for potential confounders. Because most previous studies have demonstrated a strong HPA stress axis response to be positively associated with adverse short-term outcomes [20–24], the present findings are somewhat counterintuitive. Hypothesizing that high levels of proinflammatory cytokines lead to an increase in cortisol [1–3, 37] to avoid excessive inflammation, a more pronounced initial glucocorticoid response might reflect not only the degree of stress but also the ability to generate an adequate immune response. Within this study, we demonstrated an independent correlation between admission cortisol levels and markers of inflammation (PCT and high peak CRP). From this point of view, our findings are in line with those of a previous study demonstrating a more pronounced inflammatory host response reflected by a history of chills, high body temperature, and high peak levels of CRP being associated with lower long-term mortality in patients surviving a CAP episode [38]. These results were similar to those of the Pneumonia Patient Outcomes Research Team cohort study, another large follow-up study of patients with CAP, in which researchers reported an association between the absence of fever and higher long-term mortality than among age-matched control subjects [39]. Contrarily, several studies have shown an association between worse short-term outcomes and strong inflammatory response in CAP [40–42]. On the basis of these data, it is tempting to hypothesize that survival of a clinically and biochemically pronounced CAP episode—reflected by high admission glucocorticoid levels—might mirror a more robust stress response, and, concordantly, also host defense, and thus a better general condition with lower mortality in the long term. However, a pronounced glucocorticoid response on admission might reflect, as a presumable consequence of inflammation, the ability to generate an appropriate immune response, thereby predisposing these “fitter” patients, with regard to the stress-response, to better long-term survival owing to decreased subsequent infections [43]. Further investigations are still warranted to validate these findings and determine underlying pathophysiology. Given the evidence of pronounced inflammation being associated with favorable long-term outcome in hospitalized patients with CAP, the rationale of steroid treatment in this population should be questioned.
The main strengths of this study include the well-characterized cohort of patients hospitalized for CAP, the long follow-up period of approximately 6 years, and the thorough and highly accurate biomarker measurement by liquid chromatography coupled with tandem mass spectrometry. This technique allows a proper separation and sensitive characterization of even small molecules [44]. Importantly, mass spectrometry is increasingly becoming accepted as a routine diagnostic instrument in clinical laboratories [45]. Furthermore, the large sample size and the high event number of the primary endpoint (47.3%) lead to high statistical power, which allows detecting even small differences in glucocorticoid levels.
The following limitations require consideration. First, the present study is a secondary analysis and therefore not designed primarily to perform observational biomarker outcome studies. Importantly, there was no controlling for the time point of blood sampling, because initial blood samples were taken at the time of first patient contact in the ED. However, although cortisol exhibits diurnal concentration changes, during acute illness, the circadian pattern is usually lost [5, 46, 47]. Also, the long storage of blood may have affected blood hormone levels. In addition, there was no assessment of adrenal insufficiency based on the response to injection of synthetic adrenocorticotropin. Nevertheless, previous data demonstrated a very low rate of adrenal insufficiency in patients with CAP in the absence of septic shock [23]. However, deviation of the present results by different blood sampling time points or adrenal insufficiency cannot be definitively ruled out, although this lack of standardization reflects daily clinical practice. A more standardized hormone level determination at the same time of the day in all patients would most probably have demonstrated an even higher association between glucocorticoid levels and CAP outcomes. Moreover, serial hormone measurements over the course of the disease may add valuable information.
Second, the initial trial was conducted in different secondary and tertiary care hospitals in Switzerland, and thus results may not unconditionally be applied to other geographical or institutional settings. In addition, our analysis was focused specifically on patients with CAP; further studies are required to validate these findings for other medical and surgical patient populations. Finally, because this was an observational study, it is only hypothesis-generating, and correlation does not imply a causal relationship.
Conclusions
Cortisol showed the highest association with outcome in patients with mild to severe CAP. The association of the glucocorticoid levels with adverse outcome in patients with CAP changed over time; whereas high admission levels of cortisol were independently associated with short-term adverse outcome, a more pronounced initial glucocorticoid stress response was highly associated with favorable long-term outcome. Initial 11-deoxycortisol levels showed the highest correlation with disease severity. Underlying pathophysiological aspects are still poorly examined, and future studies are needed to better understand the importance of adrenal hormones in the resolution of patients with CAP.
Key messages
Among different glucocorticoid hormones, cortisol showed the highest association with mortality in patients with CAP.
The association of glucocorticoid metabolites with outcome in CAP changed over time; whereas a more pronounced glucocorticoid stress response on initial hospital presentation was associated with higher short-term adverse outcome, increased admission hormone levels showed association with favorable long-term outcome.
Acknowledgements
We are grateful to the emergency department, medical clinic, and central laboratory staff of the University Hospital Basel and the cantonal hospitals of Aarau, Liestal, Lucerne, Muensterlingen, and the ‘Buergerspital’ Solothurn for their assistance and technical support. In particular, we thank all patients, their relatives, and all local general practitioners who participated in this study. Finally, we acknowledge the ProHOSP Study Group for their important support.
The ProHOSP Study Group includes the following persons: Ursula Schild, RN; Katharina Regez, RN; Rita Bossart, RN; Robert Thomann, MD; Claudine Falconnier, MD; Marcel Wolbers, PhD; Stefanie Neidert, MD; Thomas Fricker, MD; Claudine Blum, MD; Thomas Bregenzer, MD; Claus Hoess, MD; Heiner C. Bucher, MD; Fabian Mueller; Jeannine Haeuptle; Roya Zarbosky; Rico Fiumefreddo, MD; Melanie Wieland, RN; Charly Nusbaumer, MD; Andres Christ, MD; Roland Bingisser, MD; Kristian Schneider, RN; Brigitte Walz, PhD; Verena Briner, MD; Dieter Conen, MD; Andreas Huber, MD; Jody Staehelin, MD; Chantal Bruehlhardt, RN; Ruth Luginbuehl, RN; Agnes Muehlemann, PhD; Ineke Lambinon; Werner Zimmerli, MD; and Max Zueger, MD.
Funding
This study was supported in part by the Swiss National Science Foundation (SNSF Professorship PP00P3_150531/1) and the Research Council of the Kantonsspital Aarau (1410.000.044). The initial trial was funded by the Swiss National Science Foundation (grant SNF 3200BO-116177/1), Santé Suisse, and the Gottfried and Julia Bangerter-Rhyner Foundation. The funding organization(s) played no role in the design of the study; in the collection, analysis, and interpretation of data; or in the writing of the manuscript.
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Authors’ contributions
MCC, BM, and PS created the study concept and design, wrote the protocol, and initiated the initial ProHOSP study. MN and PS drafted the manuscript and performed statistical analyses. CS and AH performed laboratory measurements of glucocorticoids. All authors contributed to the data acquisition, interpretation and drafting of the analyses, as well as to critical review for important intellectual content, and final approval of the manuscript. PS had full access to all data in the present study and takes responsibility for the integrity of the work and the accuracy of the data analyses. MO, AK, WZ, CH, CH, and LB contributed to the data acquisition, interpretation and drafting of the analyses, as well as to critical review for important intellectual content, and final approval of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the University of Basel as well as by the local ethics committees of Aarau, Muensterlingen, Solothurn, and Luzern, all with the same ethical study number 87/06. Written informed consent was provided by all participants for the initial trial, including agreement to use their data anonymized in secondary analyses.
Abbreviations
- CAP
Community-acquired pneumonia
- CRP
C-reactive protein
- CURB-65
Confusion of new onset, blood urea nitrogen >7 mmol/L, respiratory rate ≥30 breaths per minute, systolic blood pressure <90 mmHg or diastolic blood pressure ≤60, and age ≥65 years
- ED
Emergency department
- HPA
Hypothalamic-pituitary-adrenal
- ICU
Intensive care unit
- PAOD
Peripheral arterial occlusive disease
- PCT
Procalcitonin
- PSI
Pneumonia severity index
- SBP
Systolic blood pressure
- SIRS
Systemic inflammatory response syndrome
- UHPLC
Ultra-high-performance liquid chromatography
Additional file
Baseline characteristics overall and stratified by 30-day adverse outcome, including the combined endpoint death/ICU admission in CAP. Data are presented as median [IQR] or number (percent); p values are considered statistically significant at p < 0.05. Bold values indicate statistical significance. CAP, Community-acquired pneumonia; CRP, C-reactive protein; CURB65, Confusion of new onset, blood urea nitrogen >7 mmol/L, respiratory rate ≥30 breaths per minute, systolic blood pressure <90 mmHg or diastolic blood pressure ≤60, and age ≥65 years; ICU, Intensive care unit; PAOD, Peripheral arterial occlusive disease; PCT, Procalcitonin; PSI, Pneumonia severity index; SBP, Systolic blood pressure; SIRS, Systemic inflammatory response syndrome. *Comorbidities were identified on the basis of medical records or patient report. (DOCX 21 kb)
Contributor Information
Manuela Nickler, Email: manuela_nickler@bluewin.ch.
Manuel Ottiger, Email: manuel.ottiger@gmx.net.
Christian Steuer, Email: christian.steuer@pharma.ethz.ch.
Alexander Kutz, Email: kutz.alexander@gmail.com.
Mirjam Christ-Crain, Email: mirjam.christ@usb.ch.
Werner Zimmerli, Email: werner.zimmerli@unibas.ch.
Robert Thomann, Email: endodiab.bss@bluewin.ch.
Claus Hoess, Email: claus.hoess@stgag.ch.
Christoph Henzen, Email: christoph.henzen@luks.ch.
Luca Bernasconi, Email: luca.bernasconi@ksa.ch.
Andreas Huber, Email: andreas.huber@ksa.ch.
Beat Mueller, Email: happy.mueller@unibas.ch.
Philipp Schuetz, Email: schuetzph@gmail.com.
for the ProHOSP Study Group:
Ursula Schild, Katharina Regez, Rita Bossart, Robert Thomann, Claudine Falconnier, Marcel Wolbers, Stefanie Neidert, Thomas Fricker, Claudine Blum, Thomas Bregenzer, Claus Hoess, Heiner C. Bucher, Fabian Mueller, Jeannine Haeuptle, Roya Zarbosky, Rico Fiumefreddo, Melanie Wieland, Charly Nusbaumer, Andres Christ, Roland Bingisser, Kristian Schneider, Brigitte Walz, Verena Briner, Dieter Conen, Andreas Huber, Jody Staehelin, Chantal Bruehlhardt, Ruth Luginbuehl, Agnes Muehlemann, Ineke Lambinon, Werner Zimmerli, and Max Zueger
References
- 1.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 2.Nylen ES, Müller B. Endocrine changes in critical illness. J Intensive Care Med. 2004;19(2):67–82. doi: 10.1177/0885066603259551. [DOI] [PubMed] [Google Scholar]
- 3.Müller B. Endocrine aspects of critical illness. Ann Endocrinol (Paris) 2007;68(4):290–8. doi: 10.1016/j.ando.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Schuetz P, Müller B. The hypothalamic-pituitary-adrenal axis in critical illness. Endocrinol Metab Clin North Am. 2006;35(4):823–38. doi: 10.1016/j.ecl.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, de Zegher F, Bouillon R. Clinical review 95: acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83(6):1827–34. doi: 10.1210/jcem.83.6.4763. [DOI] [PubMed] [Google Scholar]
- 6.Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30(6):1267–73. doi: 10.1097/00003246-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Jurney TH, Cockrell JL, Jr, Lindberg JS, Lamiell JM, Wade CE. Spectrum of serum cortisol response to ACTH in ICU patients: correlation with degree of illness and mortality. Chest. 1987;92(2):292–5. doi: 10.1378/chest.92.2.292. [DOI] [PubMed] [Google Scholar]
- 8.Span LF, Hermus AR, Bartelink AK, Hoitsma AJ, Gimbrere JS, Smals AG, et al. Adrenocortical function: an indicator of severity of disease and survival in chronic critically ill patients. Intensive Care Med. 1992;18(2):93–6. doi: 10.1007/BF01705039. [DOI] [PubMed] [Google Scholar]
- 9.Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283(8):1038–45. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- 10.Sam S, Corbridge TC, Mokhlesi B, Comellas AP, Molitch ME. Cortisol levels and mortality in severe sepsis. Clin Endocrinol (Oxf) 2004;60(1):29–35. doi: 10.1111/j.1365-2265.2004.01923.x. [DOI] [PubMed] [Google Scholar]
- 11.Burchard K. A review of the adrenal cortex and severe inflammation: quest of the “eucorticoid” state. J Trauma. 2001;51(4):800–14. doi: 10.1097/00005373-200110000-00033. [DOI] [PubMed] [Google Scholar]
- 12.Schein RM, Sprung CL, Marcial E, Napolitano L, Chernow B. Plasma cortisol levels in patients with septic shock. Crit Care Med. 1990;18(3):259–63. doi: 10.1097/00003246-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Arlt W, Hammer F, Sanning P, Butcher SK, Lord JM, Allolio B, et al. Dissociation of serum dehydroepiandrosterone and dehydroepiandrosterone sulfate in septic shock. J Clin Endocrinol Metab. 2006;91(7):2548–54. doi: 10.1210/jc.2005-2258. [DOI] [PubMed] [Google Scholar]
- 14.Feng JY, Liu KT, Abraham E, Chen CY, Tsai PY, Chen YC, et al. Serum estradiol levels predict survival and acute kidney injury in patients with septic shock - a prospective study. PLoS One. 2014;9(6):e97967. doi: 10.1371/journal.pone.0097967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56(10):1–120. [PubMed] [Google Scholar]
- 16.Brown PD, Lerner SA. Community-acquired pneumonia. Lancet. 1998;352(9136):1295–302. doi: 10.1016/S0140-6736(98)02239-9. [DOI] [PubMed] [Google Scholar]
- 17.File TM. Community-acquired pneumonia. Lancet. 2003;362(9400):1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos-Gallego CG, Badimon JJ. The sum of two evils: pneumonia and myocardial infarction: is platelet activation the missing link? J Am Coll Cardiol. 2014;64(18):1926–8. doi: 10.1016/j.jacc.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Santos-Gallego CG, Badimon JJ. Cardiac complications after community-acquired pneumonia. Am J Cardiol. 2016;117(2):310. doi: 10.1016/j.amjcard.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Christ-Crain M, Stolz D, Jutla S, Couppis O, Müller C, Bingisser R, et al. Free and total cortisol levels as predictors of severity and outcome in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;176(9):913–20. doi: 10.1164/rccm.200702-307OC. [DOI] [PubMed] [Google Scholar]
- 21.Kolditz M, Hoffken G, Martus P, Rohde G, Schutte H, Bals R, et al. Serum cortisol predicts death and critical disease independently of CRB-65 score in community-acquired pneumonia: a prospective observational cohort study. BMC Infect Dis. 2012;12:90. doi: 10.1186/1471-2334-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller C, Blum CA, Trummler M, Stolz D, Bingisser R, Mueller C, et al. Association of adrenal function and disease severity in community-acquired pneumonia. PLoS One. 2014;9(6):e99518. doi: 10.1371/journal.pone.0099518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolditz M, Halank M, Schulte-Hubbert B, Hoffken G. Adrenal function is related to prognosis in moderate community-acquired pneumonia. Eur Respir J. 2010;36(3):615–21. doi: 10.1183/09031936.00191709. [DOI] [PubMed] [Google Scholar]
- 24.Salluh JI, Bozza FA, Soares M, Verdeal JC, Castro-Faria-Neto HC, Lapa e Silva JR, Bozza PT. Adrenal response in severe community-acquired pneumonia: impact on outcomes and disease severity. Chest. 2008;134(5):947–54. doi: 10.1378/chest.08-1382. [DOI] [PubMed] [Google Scholar]
- 25.Nickler M, Ottiger M, Steuer C, Huber A, Anderson JB, Müller B, et al. Systematic review regarding metabolic profiling for improved pathophysiological understanding of disease and outcome prediction in respiratory infections. Respir Res. 2015;16:125. doi: 10.1186/s12931-015-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–66. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 27.Schuetz P, Christ-Crain M, Wolbers M, Schild U, Thomann R, Falconnier C, et al. Procalcitonin guided antibiotic therapy and hospitalization in patients with lower respiratory tract infections: a prospective, multicenter, randomized controlled trial. BMC Health Serv Res. 2007;7:102. doi: 10.1186/1472-6963-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzales R, Sande MA. Uncomplicated acute bronchitis. Ann Intern Med. 2000;133(12):981–91. doi: 10.7326/0003-4819-133-12-200012190-00014. [DOI] [PubMed] [Google Scholar]
- 29.Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–54. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 30.Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, Ortqvist A, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26(6):1138–80. doi: 10.1183/09031936.05.00055705. [DOI] [PubMed] [Google Scholar]
- 31.Schuetz P, Christ-Crain M, Albrich W, Zimmerli W, Mueller B, ProHOSP Study Group Guidance of antibiotic therapy with procalcitonin in lower respiratory tract infections: insights into the ProHOSP study. Virulence. 2010;1(2):88–92. doi: 10.4161/viru.1.2.10488. [DOI] [PubMed] [Google Scholar]
- 32.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 33.Grolimund E, Kutz A, Marlowe RJ, Vogeli A, Alan M, Christ-Crain M, et al. Long-term prognosis in COPD exacerbation: role of biomarkers, clinical variables and exacerbation type. COPD. 2015;12(3):295–305. doi: 10.3109/15412555.2014.949002. [DOI] [PubMed] [Google Scholar]
- 34.Alan M, Grolimund E, Kutz A, Christ-Crain M, Thomann R, Falconnier C, et al. Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: a 6-year prospective follow-up study. J Intern Med. 2015;278(2):174–84. doi: 10.1111/joim.12341. [DOI] [PubMed] [Google Scholar]
- 35.Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385(9977):1511–8. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 36.Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91(10):3725–45. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- 37.Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9(1):3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 38.Guertler C, Wirz B, Christ-Crain M, Zimmerli W, Mueller B, Schuetz P. Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J. 2011;37(6):1439–46. doi: 10.1183/09031936.00121510. [DOI] [PubMed] [Google Scholar]
- 39.Mortensen EM, Kapoor WN, Chang CC, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis. 2003;37(12):1617–24. doi: 10.1086/379712. [DOI] [PubMed] [Google Scholar]
- 40.Almirall J, Bolibar I, Toran P, Pera G, Boquet X, Balanzo X, et al. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest. 2004;125(4):1335–42. doi: 10.1378/chest.125.4.1335. [DOI] [PubMed] [Google Scholar]
- 41.Menendez R, Martinez R, Reyes S, Mensa J, Polverino E, Filella X, et al. Stability in community-acquired pneumonia: one step forward with markers? Thorax. 2009;64(11):987–92. doi: 10.1136/thx.2009.118612. [DOI] [PubMed] [Google Scholar]
- 42.Seligman R, Meisner M, Lisboa TC, Hertz FT, Filippin TB, Fachel JM, et al. Decreases in procalcitonin and C-reactive protein are strong predictors of survival in ventilator-associated pneumonia. Crit Care. 2006;10(5):R125. doi: 10.1186/cc5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mortensen EM. Potential causes of increased long-term mortality after pneumonia. Eur Respir J. 2011;37(6):1306–7. doi: 10.1183/09031936.00194110. [DOI] [PubMed] [Google Scholar]
- 44.Steuer C, Schütz P, Bernasconi L, Huber AR. Simultaneous determination of phosphatidylcholine-derived quaternary ammonium compounds by a LC-MS/MS method in human blood plasma, serum and urine samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1008:206–11. doi: 10.1016/j.jchromb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Tenori L, Oakman C, Claudino WM, Bernini P, Cappadona S, Nepi S, et al. Exploration of serum metabolomic profiles and outcomes in women with metastatic breast cancer: a pilot study. Mol Oncol. 2012;6(4):437–44. doi: 10.1016/j.molonc.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van den Berghe GH. Acute and prolonged critical illness are two distinct neuroendocrine paradigms. Verh K Acad Geneeskd Belg. 1998;60(6):487–520. [PubMed] [Google Scholar]
- 47.Bartanusz V, Corneille MG, Sordo S, Gildea M, Michalek JE, Nair PV, et al. Diurnal salivary cortisol measurement in the neurosurgical-surgical intensive care unit in critically ill acute trauma patients. J Clin Neurosci. 2014;21(12):2150–4. doi: 10.1016/j.jocn.2014.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.


