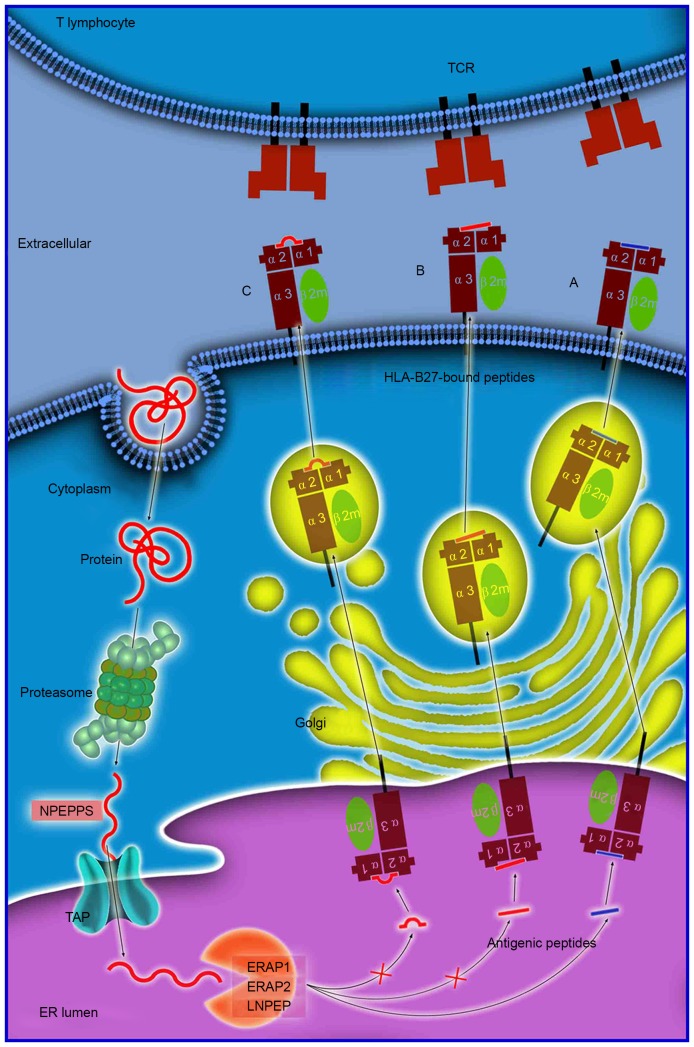
Figure 1.
Antigen processing and presentation of peptides of various sizes. Antigen processing and presentation is a sequenced process. Numerous proteins are initially degraded into peptide fragments of up to 25 amino acids in length by the multi-unit proteasome complex followed by NPEPPS. TAP preferentially transports antigen peptides of 8–16 residues into the ER. N-terminal extended precursors will be further cleaved by ERAP1/ERAP2/LNPEP into oligopeptides of 8 or 9 residues, which is the optimal length for binding to HLA-B27. The peptides subsequently (A) enter the Golgi apparatus for generation of mature epitopes. However, various longer peptides may bind to HLA-B27, where they reside in the peptide groove of the HLA-B27 with (B) a protruding C-terminus, or (C) a bulge in the center. These HLA-B27-bound peptides may be highly immunogenic and may stimulate an extremely biased T-cell response repertoire. NPEPPS, aminopeptidase puromycin sensitive; TAP, transporter, ATP-binding cassette subfamily B member; HLA, human leukocyte antigen; ER, endoplasmic reticulum; ERAP1/2, endoplasmic reticulum aminopeptidase 1/2; LNPEP, leucyl cystinyl aminopeptidase; TCR, T-cell receptor.