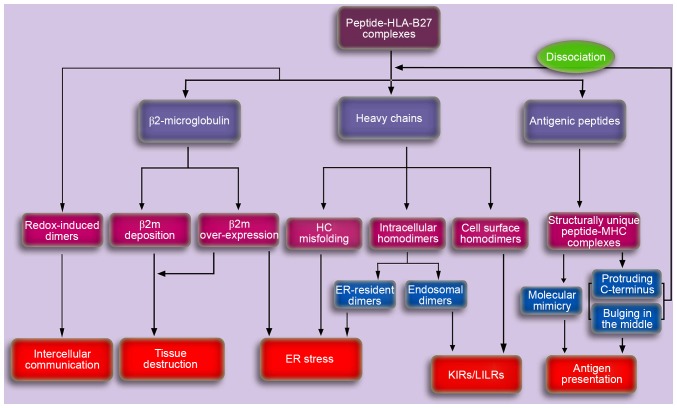
Figure 3.
A schematic depicting the potential pathogenesis of AS caused by HLA-B27. Aberrant processing and presentation of structurally unique peptides were initially proposed to explain the potential pathogenesis of AS. Cell surface HLA-B27 dimers may be recognized by various immunoreceptors and may be important in the pathogenesis of autoimmune disorders. Accumulation of proteins in the ER, including ER-resident dimers, misfolded HCs and β2m may result in the ER stress response, thereby activating the unfolded protein response, which is associated with cytokine dysregulation. In addition, the accumulating β2m in synovia for dissociation and/or overexpression, may induce the synthesis and secretion of proteins involved in tissue destruction, thus leading to AS. The exosomal fully-folded HLA-B27 dimers may be important in the pathogenesis of AS via intercellular communication. AS, ankylosing spondylitis; HLA, human leukocyte antigen; HC, heavy chain; KIR, killer cell immunoglobulin-like receptor; LILR, leucocyte immunoglobulin-like receptor; ER, endoplasmic reticulum; MHC, major histocompatibility complex; β2m, β2microglobulin.