Abstract
Teriparatide is a drug that is used to increase bone remodeling, formation, and density for the treatment of osteoporosis. We present three cases of patients with a femoral insufficiency fracture. The patients were administered teripatatide in an attempt to treat severe osteoporosis and to enhance fracture healing. We found several radiographic features around the femoral fractures during the healing period. 1) Callus formation was found at a very early stage in the treatment. Teriparatide substantially increased the unusually abundant callus formation around the fracture site at 2 weeks. Moreover, this callus formation continued for 8 weeks and led to healing of the fracture. 2) Abundant callus formation was found circumferentially around the cortex with a ‘cloud-like’ appearance. 3) Remodeling of the teriparatide-induced callus formation was found to be part of the normal fracture healing process. After 1 year, normal remodeling was observed on plain radiographs. These findings indicate that teriparatide can be used as an adjuvant therapy in the management of femoral insufficiency fractures.
Keywords: Teriparatide, Femoral fracture, Callus formation, Remodeling
Highlights
-
•
Radiographic features of teriparatide-induced healing of femoral fractures were assessed.
-
•
Teriparatide accelerated and enhanced fracture healing of femoral fractures
-
•
Teriparatide-induced fracture healing was followed by a normal fracture healing process.
-
•
Teriparatide can be used as an adjuvant therapy in the management of femoral insufficiency fractures.
1. Introduction
Teriparatide is a synthetic polypeptide hormone that contains the 1–34 amino acid fragment of the human parathyroid hormone. It has been shown to increase bone remodeling, formation, and density for the treatment of osteoporosis, and it reduces the risk of vertebral and nonvertebral fractures (Neer et al., 2001, Jiang et al., 2003). Moreover, it has been shown that teriparatide also accelerates fracture healing by improving the biomechanical properties of the fracture callus, increasing bone remodeling (Bukata and Puzas, 2010, Reynolds et al., 2009). This effect has been observed in several clinical case reports and prospective randomized control studies (Aspenberg et al., 2010, Peichl et al., 2011); however, the radiographic features of teriparatide-induced fracture healing have not been precisely described. We report the radiographic features of teriparatide-induced healing of femoral insufficiency fractures, including periprosthetic fractures.
2. Case reports
2.1. Case 1
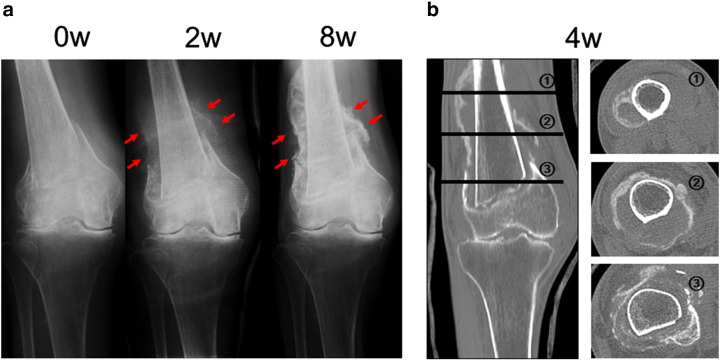
An 88-year-old woman sustained a supracondylar fracture of her right femur after falling from the bed. She had a previous history of thoracic and lumbar spine multiple compression fractures, which were treated conservatively. She had no history of treatment for osteoporosis. We started to treat this fracture conservatively and initiated administration of teriparatide (20 μg subcutaneous injection daily) in an attempt to treat severe osteoporosis and to enhance fracture healing. A plain radiograph after 2 weeks showed obvious callus formation around the fracture site, and after 8 weeks, abundant ‘cloud-like’ bridging callus formation was observed (Fig. 1a). Callus formation with a ‘cloud-like’ appearance was clearly visualized around the fracture site by CT scan taken at 4 weeks (Fig. 1b). We allowed the patient to start range-of-motion exercises for the right knee joint after 5 weeks and partial weight bearing after 7 weeks. No side effects attributable to the drug were observed during treatment.
Fig. 1.
Case 1. An 88-year-old woman who sustained a supracondylar fracture of the right femur. (a) Anteroposterior radiograph of the right knee soon after the fracture, and 2 weeks and 8 weeks after initiation of teriparatide therapy. Obvious callus formation was visualized around the fracture site was observed at 2 and 8 weeks after treatment (arrow). (b) CT scan of the right knee taken 4 weeks after initiation of teriparatide therapy.
2.2. Case 2
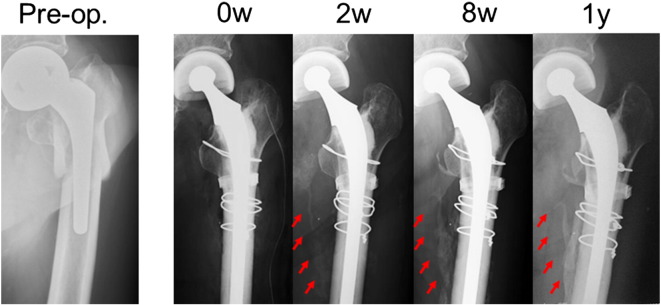
A 96-year-old woman sustained a right periprosthetic femoral fracture of type B1 (according to the Vancouver classification (Duncan and Masri, 1995)) after she fell and landed her right side. She had previously undergone a right bipolar hemiarthroplasty 10 years earlier for a femoral neck fracture and had been ambulating well prior to her fall. Medical history was significant for diabetes mellitus. She had a previous fracture of left femur, which was treated with surgical treatment. She had no history of treatment for osteoporosis. This periprosthetic fracture was treated with locking compression plate fixation. After surgery, we initiated administration of teriparatide to treat severe osteoporosis and to accelerate fracture healing. Two weeks after initiation of teriparatide therapy, a plain radiograph showed obvious callus formation around the fracture site, and after 8 weeks, abundant callus formation was observed (Fig. 2a). Again, callus formation with a ‘cloud-like’ appearance was observed around the fracture site by CT scan taken at 4 weeks (Fig. 2b). After 1 year, the initial callus diminished its volume and replaced by a hard bony callus. We allowed the patient to start partial weight bearing after 4 weeks. No side effects attributable to the drug were observed during treatment.
Fig. 2.
Case 2. A 96-year-old woman who sustained a periprosthetic femoral fracture of the right femur. (a) Anteroposterior radiograph of the right femur taken preoperatively, postoperatively, and 2 weeks, 8 weeks, and 1 year after initiation of teriparatide therapy. Obvious callus formation around the fracture site was observed at 2 and 8 weeks after treatment (arrow). After 1 year, remodeling of the fracture callus was complete (arrow). (b) CT scan of the right knee taken 4 weeks after initiation of teriparatide therapy.
2.3. Case 3
A 78-year-old man sustained a periprosthetic femoral fracture of Vancouver type B2 after he fell and landed on his left side. He had previously undergone a left bipolar hemiarthroplasty 9 years earlier to treat a femoral neck fracture and had been ambulating well. He had a previous history of thoracic and lumbar spine multiple compression fractures, which were treated conservatively. He had no history of treatment for osteoporosis. The patient was treated with stem revision using a cemented long stem and cerclage wiring. After surgery, we administered teriparatide. From 2 weeks after initiation of teriparatide therapy, a plain radiograph showed obvious callus formation, and after 8 weeks, abundant callus formation was observed. After 1 year, the initial callus diminished its volume and replaced by a hard bony callus (Fig. 3). No side effects attributable to the drug were observed during treatment.
Fig. 3.
Case 3. A 78-year-old man who sustained a periprosthetic femoral fracture of the left femur. Anteroposterior radiograph of the left femur taken preoperatively, postoperatively, and 2 weeks, 8 weeks, and 1 year after initiation of teriparatide therapy. Obvious callus formation around the fracture site was observed at 2 and 8 weeks after treatment (arrow). After 1 year, remodeling of the fracture callus was complete (arrow).
3. Discussion
Our study is among the first to report on the radiographic features of teriparatide-induced healing of femoral insufficiency fractures. Most previous case reports of teriparatide-induced fracture healing dealt with the nonunion or delayed union of fractures (Chintamaneni et al., 2010, Tamai et al., 2013, Ochi et al., 2013). Aspenberg et al described a prospective, randomized, double blind study in postmenopausal women who underwent conservative treatment for distal radial fractures (Aspenberg et al., 2010). However, these authors did not precisely describe the radiographic features of the callus formation around the radial fracture.
We identified several radiographic features around the femoral fractures during the fracture healing period. First, the callus formation was found at a very early stage in the treatment. Our cases commenced treatment with teriparatide 1 day after surgery or the initiation of conservative treatment. A plain radiograph at 2 weeks showed that treatment with teriparatide substantially increased unusually abundant callus formation around the fracture site. In femoral shaft fractures treated with intramedullary nailing, the callus appeared at a mean of 3.9 weeks after surgery (Yamji et al., 2002). This result suggests that the callus formation after teriparatide therapy was approximately 2 weeks more early than normal healing. Moreover, this callus formation progressed for 8 weeks and led to healing of the fracture. This finding is supported by several previous reports that teriparatide enhances the early proliferative response of chondroprogenitor and osteoprogenitor cells in fracture healing (Barnes et al., 2008, Nakajima et al., 2002). In general, it takes approximately 12 weeks for a femoral shaft fracture to heal. A callus formation is seen on radiograph by 6 weeks after locked plating of distal femur fractures (Lujan et al., 2010). In the Vancouver type B periprosthetic femoral fracture treated with osteosynthesis or stem revision it takes 4 to 12 months for fracture healing (Pavlou; Corten et al., 2009). Some authors have shown that teriparatide accelerates fracture healing by improving the bone quality of fracture callus and by increasing endochondral and intramembranous ossification and bone remodeling in an animal model (Nakazawa et al., 2005). Aspenberg et al. also showed that teriparatide had a strong effect on early callus formation in distal radial fractures (Aspenberg and Johansson, 2010). The increased amount of callus formation in the early phase of fracture healing can have a significant clinical benefit in some difficult fracture cases (Yamji et al., 2002, Aspenberg and Johansson, 2010).
Second, abundant callus formation was found circumferentially around the femoral cortex with a ‘cloud-like’ appearance on plain radiographs and CT scans. This type of callus formation around the femoral fracture site is unusual without teriparatide treatment. A plain radiograph showed the presence of bone bridges between the fragments. We also observed that these bone bridges were found circumferentially around the cortex.
Third, although this radiographic appearance of unusually abundant callus formation resembled heterotopic ossification or myositis ossificans, remodeling of the callus formation was found to be part of the normal fracture healing process. After 1 year, the initial callus diminished its volume and replaced by a hard bony callus in cases 2 and 3 (Fig. 2, Fig. 3). Our findings indicated that cloud-like callus formation was normal secondary fractures healing, and not the abnormal soft tissue calcification. These results suggest that the enhanced early fracture healing associated with treatment with teriparatide was followed by a normal fracture healing process. Heterotopic ossification is abnormal formation of mature bone within extraskeletal soft tissues where bone does not normally exist and is observed most frequently in the hip joint (Garland, 1991, Subbarao and Garrison, 1999). Heterotopic ossification causes considerable morbidity due to swelling, pain, and loss of range of motion (Naraghi et al., 1996, Thomas, 1992). In our cases, clinical examination showed no significant restriction in the range of motion of the hip and knee joints, no swelling, and an ability to walk without pain at the last follow-up. Furthermore, radiographic assessment showed that remodeling of the callus induced by teriparatide proceeded in the same manner as normal fracture healing. Our findings suggest that teriparatide-induced fracture healing seems to occur within the normal physiological response to bone fracture.
Our cases showed some clinical features that the fractures occurred from minor trauma in elderly patients who had comorbidities that predisposed them to osteoporosis. In particular, the postmenopausal women (cases 1 and 2) had significant osteoporosis of the femur. These patients had never been treated for osteoporosis. These insufficiency fractures occurred in the femoral diaphysis and metaphysis. Based on their radiographic appearance during the healing process induced by teriparatide, these femoral fractures appear to be different from distal radius fractures. Tamai et al. reported the accelerated healing of a femoral shaft fracture (Tamai et al., 2013). This case showed that callus formation was observed 2 weeks after teriparatide treatment and abundant callus formation was found, similar to our cases. A prospective, randomized, double blind study of teriparatide treatment in distal radial fractures showed significantly accelerated fracture healing; however, the periosteal callus formation was not so abundant in the distal radius (Aspenberg et al., 2010).
Femoral insufficiency fractures around a prosthesis are one of the most catastrophic complications of osteoporosis and are often difficult to treat (Pavlou et al., 2011). Moreover, osteoporotic fractures in the elderly make it difficult to achieve fracture healing. The positive effects of teriparatide on fracture healing 2 weeks after surgery were observed in our cases. The use of teriparatide as an adjuvant therapy for fracture healing appears to be a promising form of treatment.
There are several limitations to this study. This small case series was retrospective in design and the follow up period was short. The lack of a patient control group for comparison illustrates the need for a prospective randomized control trial to determine whether there is a role for teriparatide therapy to enhance fracture healing in cases where the natural healing process might be compromised.
Conflict of interest
Youngwoo Kim, Chiaki Tanaka, Hiroshi Tada, Hiroshi Kanoe, and Takaaki Shirai declare that they have no conflict of interest.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
References
- Aspenberg P., Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop. 2010;81:234–236. doi: 10.3109/17453671003761946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenberg P., Genant H.K., Johansson T., Nino A.J., See K., Krohn K., García-Hernández P.A., Recknor C.P., Einhorn T.A., Dalsky G.P., Mitlak B.H., Fierlinger A., Lakshmanan M.C. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010 doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- Barnes G.L., Kakar S., Vora S., Morgan E.F., Gerstenfeld L.C., Einhorn T.A. Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J. Bone Joint Surg. Am. 2008;90(Suppl. 1):120–127. doi: 10.2106/JBJS.G.01443. [DOI] [PubMed] [Google Scholar]
- Bukata S.V., Puzas J.E. Orthopedic uses of teriparatide. Curr. Osteoporos. Rep. 2010;8:28–33. doi: 10.1007/s11914-010-0006-3. [DOI] [PubMed] [Google Scholar]
- Chintamaneni S., Finzel K., Gruber B.L. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporos. Int. 2010 doi: 10.1007/s00198-009-1061-4. [DOI] [PubMed] [Google Scholar]
- Corten K., Vanrykel F., Bellemans J., Frederix P.R., Simon J.-P., Broos P.L.O. An algorithm for the surgical treatment of periprosthetic fractures of the femur around a well-fixed femoral component. J. Bone Joint Surg. (Br.) 2009;91:1424–1430. doi: 10.1302/0301-620X.91B11.22292. [DOI] [PubMed] [Google Scholar]
- Duncan C.P., Masri B.A. Fractures of the femur after hip replacement. Instr. Course Lect. 1995;44:293–340. [PubMed] [Google Scholar]
- Garland D.E. A clinical perspective on common forms of acquired heterotopic ossification. Clin. Orthop. Relat. Res. 1991;13–29 [PubMed] [Google Scholar]
- Jiang Y., Zhao J.J., Mitlak B.H., Wang O., Genant H.K., Eriksen E.F. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2003 doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- Lujan T.J., Henderson C.E., Madey S.M., Fitzpatrick D.C., Marsh J.L., Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J. Orthop. Trauma. 2010;24:156–162. doi: 10.1097/BOT.0b013e3181be6720. [DOI] [PubMed] [Google Scholar]
- Nakajima A., Shimoji N., Shiomi K., Shimizu S., Moriya H., Einhorn T.A., Yamazaki M. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34) J. Bone Miner. Res. 2002;17:2038–2047. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- Nakazawa T., Nakajima A., Shiomi K., Moriya H., Einhorn T.A., Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1–34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37:711–719. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Naraghi F.F., DeCoster T.A., Moneim M.S., Miller R.A., Rivero D. Heterotopic ossification. Orthopedics. 1996;19:145–151. doi: 10.3928/0147-7447-19960201-10. [DOI] [PubMed] [Google Scholar]
- Neer R.M., Arnaud C.D., Zanchetta J.R., Prince R., Gaich G.A., Reginster J.Y., Hodsman A.B., Eriksen E.F., Ish-Shalom S., Genant H.K., Wang O., Mitlak B.H. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001 doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Ochi K., Ikari K., Naomi A., Momohara S. Administration of teriparatide treatment for a challenging case of nonunion of periprosthetic fracture after total knee arthroplasty. Arch. Osteoporos. 2013;8:159. doi: 10.1007/s11657-013-0159-7. [DOI] [PubMed] [Google Scholar]
- Pavlou G., Panteliadis P., Macdonald D., Timperley J.A., Gie G., Bancroft G., Tsiridis E. A review of 202 periprosthetic fractures—stem revision and allograft improves outcome for type B fractures. Hip Int. 2011;21:021–029. doi: 10.5301/hip.2011.6301. [DOI] [PubMed] [Google Scholar]
- Peichl P., Holzer L.A., Maier R., Holzer G. Parathyroid hormone 1–84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J. Bone Joint Surg. Am. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- Reynolds D.G., Shaikh S., Papuga M.O., Lerner A.L., O'Keefe R.J., Schwarz E.M., Awad H.A. muCT-based measurement of cortical bone graft-to-host union. J. Bone Miner. Res. 2009;24:899–907. doi: 10.1359/JBMR.081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao J.V., Garrison S.J. Heterotopic ossification: diagnosis and management, current concepts and controversies. J. Spinal Cord Med. 1999;22:273–283. doi: 10.1080/10790268.1999.11719580. [DOI] [PubMed] [Google Scholar]
- Tamai K., Takamatsu K., Kazuki K. Successful treatment of nonunion with teriparatide after failed ankle arthrodesis for Charcot arthropathy. Osteoporos. Int. 2013;24:2729–2732. doi: 10.1007/s00198-013-2367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.J. Heterotopic bone formation after total hip arthroplasty. Orthop. Clin. North Am. 1992;23:347–358. [PubMed] [Google Scholar]
- Yamji T., Ando K., Nakamura T., Washimi O., Terada N. 2002. Femoral shaft fracture callus formation after intramedullary nailing: a comparison of interlocking and Ender nailing; pp. 472–476. (Journal of Orthopaedic Science). [DOI] [PubMed] [Google Scholar]