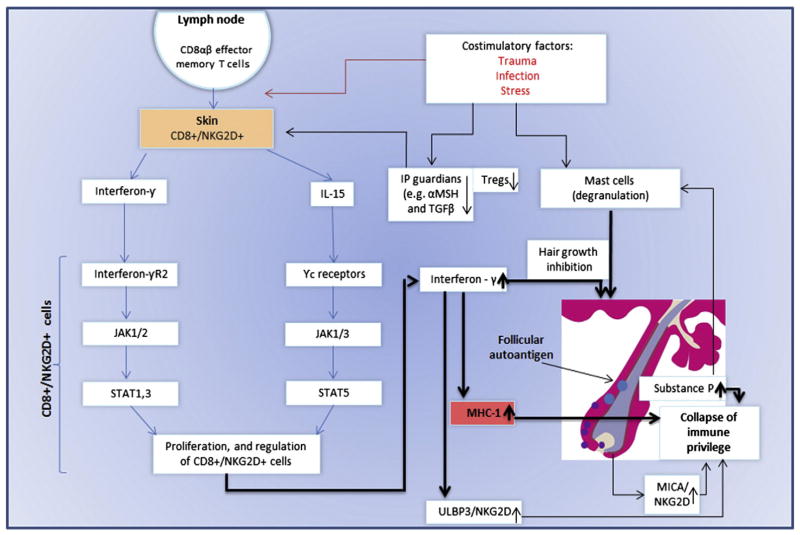
Fig. 2. The pathogenesis of AA.
CD8αβ + effector memory t as well as NK cells expressing NKG2D produce a large amount of IFN-γ in regional lymph nodes in of affected AA C3H/HeJ mouse model [68]. These cells were also detected in human lesional areas of AA [18,68]. Affymetrix microarray analysis demonstrated INF response gene and IL-15 (74). IL-15R is expressed on cd8 + cells surrounding the human AA [68], and serve as a growth factor to effectors’ CD8 + and NK cells [74,131]. The common gamma chain (γc) (expressed by il-15R) signals through Janus kinase (JAK) 1 and 3, whereas INF-γ through JAK 1 and 2. JAKs activation leads to the phosporylation and activation of STATS which will translocate to the nucleus. This will lead to proliferation and activation of preexisting autoreacive CD8+ T cells [68]. These cells produce large amount of INF-γ which will induce MHC class I in hair follicle leading to the IP collapse and the ability of the CD8+ cells and NK cells, to attack the hair follicle and induce AA. This process may occur in AA patients with genetic background that may also be predisposed to environmental triggers such as trauma, infection and stress (with the release of substance P). Furthermore, in both mice and man, a decrease number of T regulatory cells (Tregs) were observed [132]. T-regs are known to be a crucial player in the preventing autoimmune phenomena. Recently, an interaction of perifollicular mast cells and CD8+ cells was observed. This interaction may impact IP maintenance, or, under inflammatory condition, IP collapsed.