Figure 6.
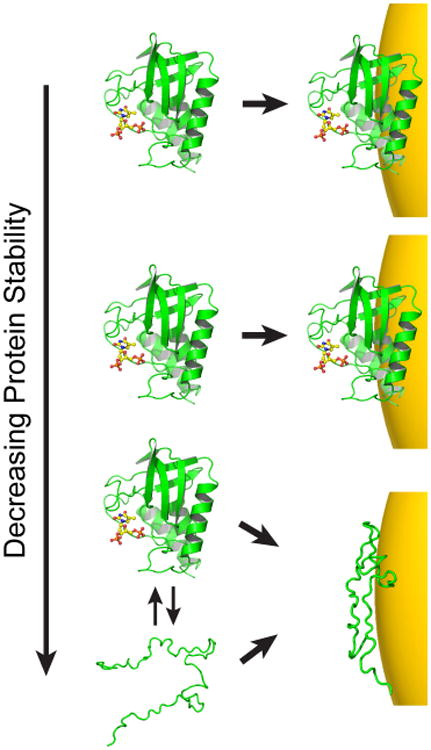
Hypothesized relationship between stability and AuNP adsorption. On the basis of the data presented here, stable proteins appear to remain globular when adsorbed, but the unstable drkN SH3 domain exhibits anomalous binding. Initially, as stability is lowered, protein adsorption may not be affected (top two panels). As folding stability continues to decrease, however, a protein may interact differently with the AuNP surface. This interaction may be modulated by direct binding of the unfolded state, or deformation of the folded state once bound. As a result, the apparent binding capacity can no longer be predicted by the native state structure.
