Fig. 6. Binding by either SH2 domain allosterically activates SHP-1, but the reach parameter is larger for N-SH2 binding.
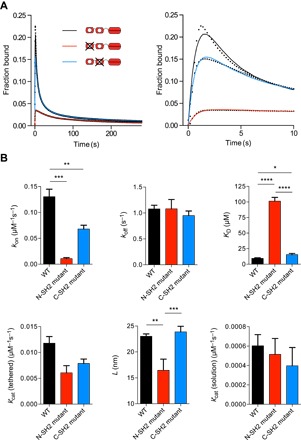
(A) SPR traces (black dots) and MPDPDE model fits (solid lines) of the N- and C-terminal SH2 domain binding–null mutants and wild-type SHP-1 injected over PEG28-ITIM. (B) Average fit parameters for wild-type (WT) SHP-1 (n = 15), N-terminal SH2 (N-SH2) mutant SHP-1 (n = 3), and C-terminal SH2 (C-SH2) mutant SHP-1 (n = 9) show weak binding and reduced reach when SHP-1 binds via the C-terminal SH2 domain compared to the N-terminal SH2 domain, but allosteric activation is observed in both cases. Two-way ANOVA with a Bonferroni multiple comparison correction is used to determine the P values (****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05).
