Figure 1. Contraction of the T6SS Results in the Translocation of Effector Proteins.
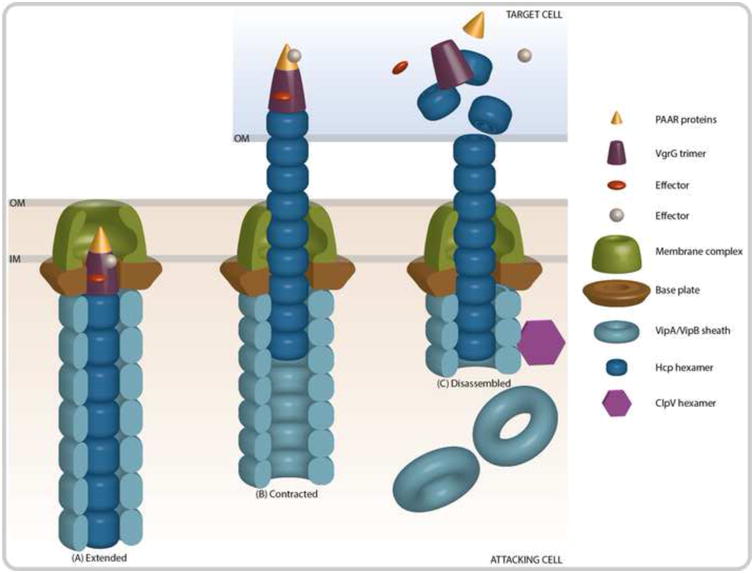
(A) The membrane complex is comprised of both a baseplate structure TssEFGK (HsiF and VasABE in V. cholerae), as well as membrane anchoring components TssJLM (VasDFK in V. cholerae). The outer sheath (VipA/VipB) and inner tube (Hcp) proteins polymerize to form an extended tube that is assembled onto VgrG and PAAR-motif proteins at the cytoplasmic side of the membrane complex. Effector proteins are recruited to the PAAR or VgrG proteins. (B) After assembly, the T6SS complex remains stable until an unknown signal results in the contraction of the outer sheath and the propulsion of the inner tube into a neighboring cell. (C) Upon translocation of the inner tube, tip, and effector complex, the effector proteins can exhibit their toxic activity. Concurrently, the ATPase ClpV disassembles the outer sheath so that the components can be recycled.
