Fig. 6.
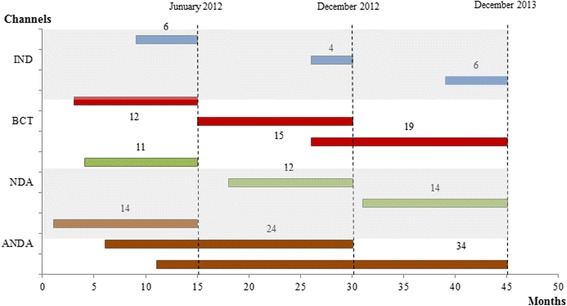
Average waiting time for technical review of chemical drugs. 1. Data source: 2013 China Drug Review Annual Report. 2. Figure 6 describes the average waiting time for technical review of chemical drugs in four channels, including Investigational New Drug (IND), New Drug Application (NDA), bridging clinical trial (abbreviated as BCT in Fig. 1) and Abbreviated New Drug Application (ANDA). Waiting time is measured in month and calculated as the difference between CDE’s reception date (the day CDE receives drug evaluation request of certain applications from CFDA) and technical review starting time. The January 2012, December 2012 and December 2013 are three time points that CDE commences technical review of certain applications
