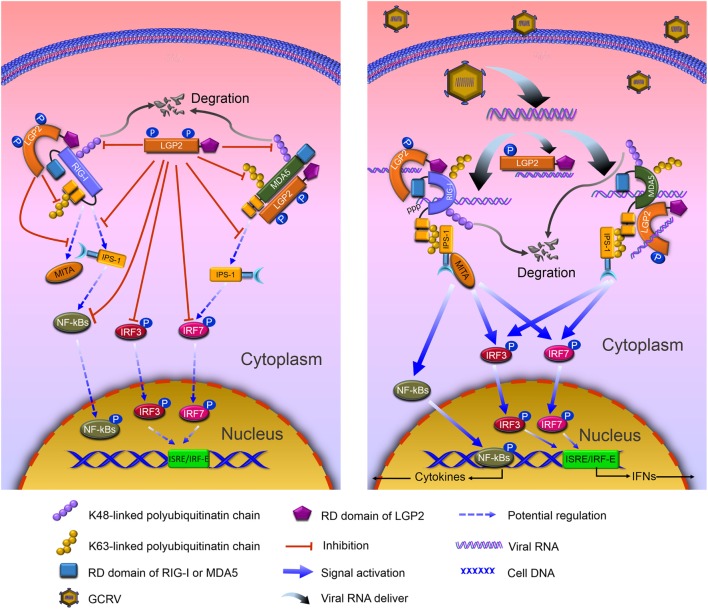
Figure 9.
Model of negative role of laboratory of genetics and physiology 2 (LGP2) in modulating retinoic acid-inducible gene I (RIG-I)- and melanoma differentiation-associated gene 5 (MDA5)-mediated antiviral signaling in grass carp. Left: in resting state, LGP2 binds Helicase and repressor domains (RDs) of RIG-I, but leaves the CARDs to form an anti-inhibited state with Helicase, which weakens binding with downstream adaptor IFN-β promoter stimulator 1 (IPS-1). Besides binding to Helicase and CARDs domains, LGP2 competitively interacts with the CARDs of MDA5 that represses MDA5 to form anti-inhibited comformation and interaction with IPS-1. Meanwhile, LGP2 suppresses K63-linked polyubiquitination of RIG-I CARDs and MDA5 Helcase or RD domains. Consequently, signal transductions from RIG-I and MDA5 to IPS-1 and mediator of IRF3 activation (MITA) are inhibited. Furthermore, LGP2 restrains phosphorylation and expressions of IRF3 and IRF7, and the subsequent signals of NF-κBs and IFNs. Concomitantly, LGP2 suppresses the degradation of RIG-I and MDA5 through inhibiting K48-linked polyubiquitination of RIG-I and MDA5, which ensures the basal levels of RIG-I and MDA5 for subsequent antiviral activation. Right: grass carp reovirus (GCRV) infection induces the activation of RLR signals, yet vanishes LGP2-induced inhibiton. dsRNAs derived from GCRV facilitate conformational change of RIG-I and MDA5. Activated RIG-I and MDA5 release the CARDs, which interact with the CARD of IPS-1. IPS-1, then associates with MITA, and activates downstream signals via NF-κBs and IRF3/IRF7-IFNs pathways. However, in GCRV invading cells, LGP2 remains to interact with RIG-I and MDA5, disappears Thr-phosphorylation, which may contribute to the derepression for RLR-mediated activation.