Table 2. Parameters for analysis of the L process.
| (a) amino acid in water | (b) amino acid in collagen aqueous solution | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| amino acid | x(%) | τL(ps) | τa(ps) | a[Å] | b[Å] | εs | 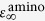 |
ccollagen(wt%) | τa (ps) |
| glycine | 3.0 | 72 | 74 | 3.67 | 2.11 | 120 | 91.3 | 4.0 | 74 |
| β-alanine | 3.0 | 107 | 108 | 4.30 | 2.21 | 135 | 88.2 | 4.0 | 112 |
| L-serine | 3.0 | 123 | 114 | 3.93 | 2.66 | 123 | 83.3 | 0.4 | 120 |
| L-arginine | 1.0 | 309 | 282 | 6.30 | 2.68 | 114 | 74.4 | 0.4 | 309 |
(a) Observed dielectric relaxation time of the L process, τL, in the aqueous solutions of amino acids and calculated relaxation time, τa, assuming that the origin of the L process is rotational diffusion of the amino acid molecule. All the parameters used to calculate the values of τa with Perrin’s equation (Eq. 2) are also listed in this table. In our calculations, we did not account for the effect of hydrated water surrounding the amino acid molecule because the volume occupied by such water molecules is not well defined. Clearly, the values of observed relaxation times, τL, for glycine and β-alanine are similar to those of τa. However, the values of τa for L-serine and L-arginine are somewhat less than those obtained experimentally. Because these amino acids have long and charged residuals, it can be assumed that such amino acids are surrounded by a larger amount of hydrated water compared to glycine and β-alanine. For this reason, the experimental relaxation times are greater than the relaxation times estimated using Eq. 2. (b) Collagen concentration, ccollagen, and observed relaxation time of the L process, τL, in the aqueous solutions of collagen with an amino acid. Note that the values of τL in (b) and those in (a) are identical. This fact suggests that the local viscosity of the aqueous solution with collagen is the same as that of pure water.
