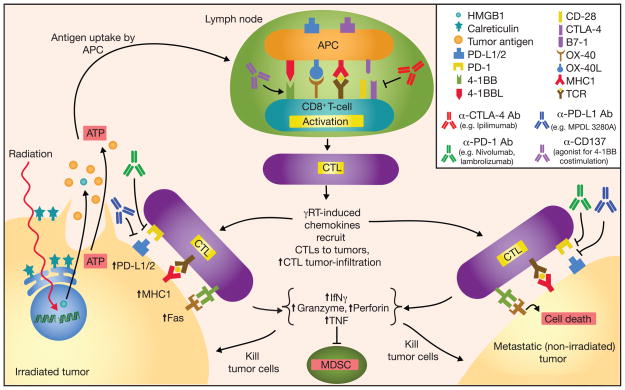
Figure 1.
Schematic diagram outlining the antitumor activity and abscopal effect in combining checkpoint inhibitors with radiation-induced immune response. Radiation induces DNA damage and tumor cell death by promoting tumor cell expression of Fas and MHC class I; dying tumor cells release ATP, tumor antigens, and danger signals such as HMGB1 and calreticulin. Radiation also increases tumor cell expression of PD-L1 and secretion of TGF-β, and suppression of CD4+ Treg. Tumor antigens captured by antigen-presenting cells (APC) are processed and presented on MHC class I molecules in the draining lymph node to tumor antigen-specific T cells in conjunction with co-stimulation to promote activation and proliferation. CTLA-4 can bind B7-1 to downregulate T-cell activation. Activated CTLs leave the lymph node, follow inflammatory chemokines, and migrate to tumor sites. PD-L1 and PD-1 can interact to suppress CTL activation; various α–CTLA-4, α–PD-L1, and α-PD-1 mAbs have been developed and used successfully in cancer immunotherapies. CTL antitumor activity includes secretion of INFγ and TNF, suppression of myeloid derived suppressor cells (MDSC), expression of perforin and granzyme, and activation of Fas ligand-mediated tumor cell apoptosis.