Fig. 3.
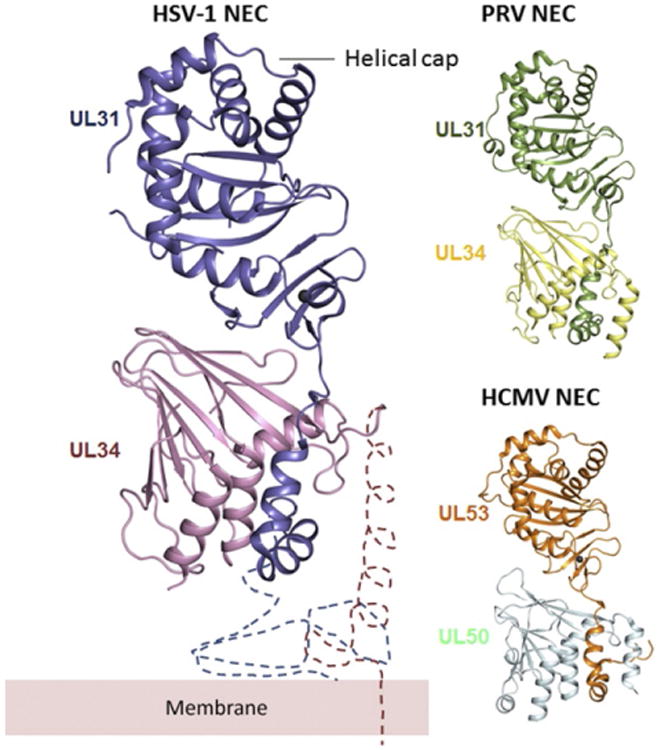
Comparison of HSV-1, PRV, and HCMV NEC crystal structures. All structures are shown in the same orientation. UL31 and UL34 form an elongated complex, with UL31 wrapping its N-terminal hook around UL34. The two molecules interact extensively, which implies high binding affinity. The membrane-proximal end is located at the bottom of the heterodimer in this orientation. The regions important for membrane interaction are missing from the structure and are indicated schematically, along with the membrane, only for HSV-1 but are expected to have a similar location in PRV and HCMV NEC. The C-terminal helix (α4) in HSV-1 UL34 was not resolved in the crystal structure. Overall, NEC structures from three different viruses are very similar, but the relative orientations of UL31 and UL34 are slightly different in HCMV (PDB ID: 4ZXS, 4Z3U, and 5DOB).
