FIG. 2.
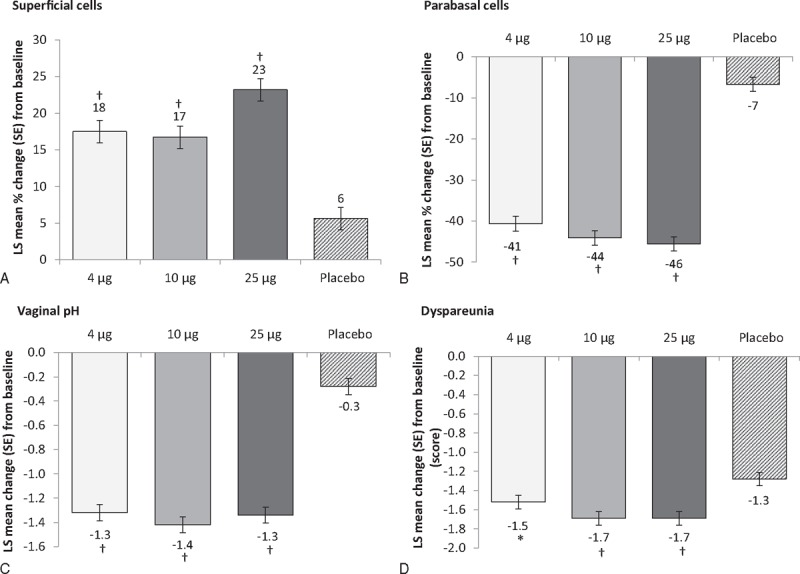
Co-primary endpoints: change from baseline to week 12 in (A) percentage of superficial cells; (B) percentage of parabasal cells; (C) vaginal pH units, and (D) dyspareunia severity scores with three doses of TX-004HR compared with placebo. ∗P < 0.05; †P < 0.0001 versus placebo (MITT population, n = 747). LS, least square; MITT, modified intent-to-treat.
