FIG. 3.
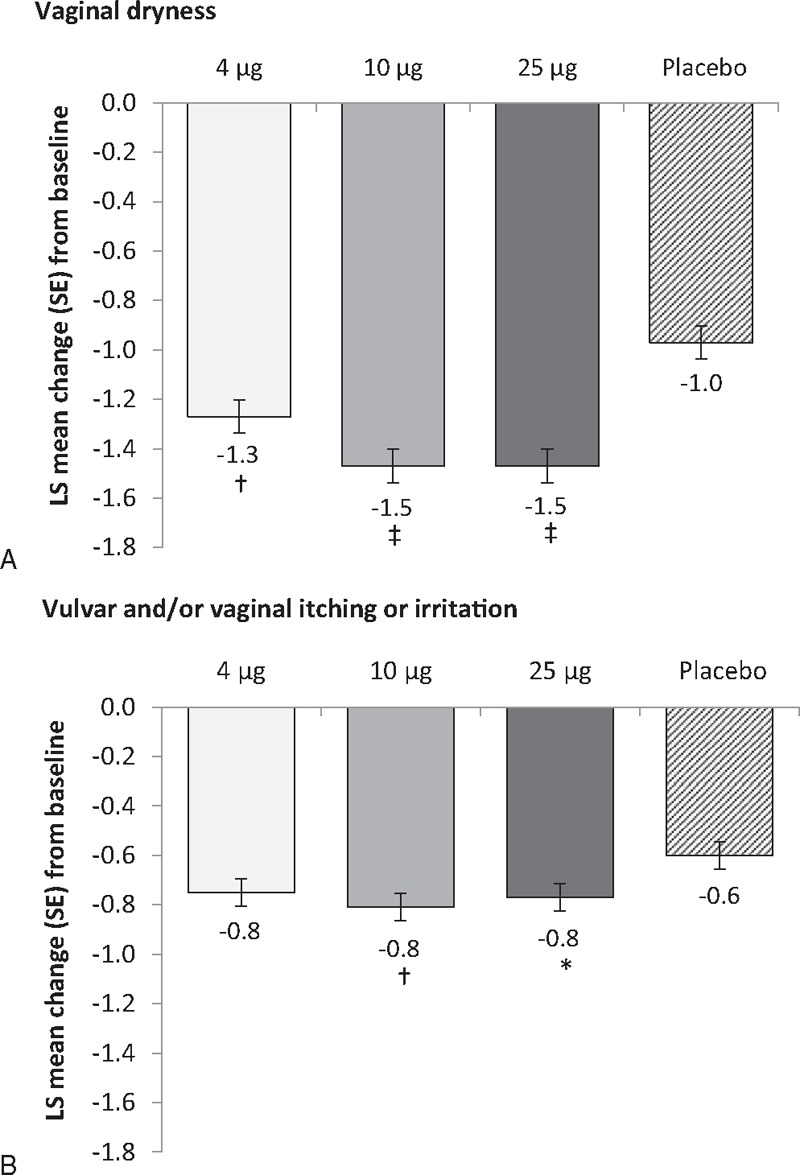
Effect of each of three doses of TX-004HR at 12 weeks compared with placebo on the secondary endpoints of (A) severity of vaginal dryness and (B) severity of vulvar and/or vaginal itching or irritation (MITT population, n = 747). ∗P < 0.05; †P < 0.01; ‡P < 0.0001 versus placebo. LS, least square; MITT, modified intent-to-treat.
