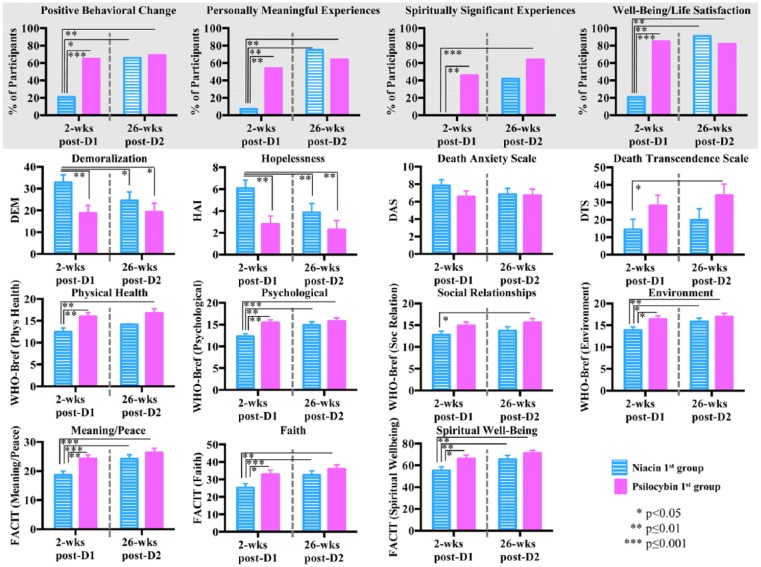
Figure 6.
Secondary outcome measures: existential distress, quality of life, spirituality, persisting effects attributed to psilocybin administration.
(Top) Percentage of participants that reported ‘among the top 5’ or ‘the single most’ personally meaningful and spiritually significant experiences, ‘moderate’, ‘strong’ or ‘extreme’ positive behavioral change, and ‘increased moderately’ or ‘increased very much’ wellbeing or life satisfaction on the Persisting Effects Questionnaire (PEQ). Asterisks indicate significance level of comparison to the niacin first group at 2 weeks post-dose 1. There were no significant differences between the psilocybin first group at 2 weeks post-dose 1 versus the psilocybin first group at 26 weeks post-dose 2. (Bottom) Secondary measures of cancer-related existential distress (DEM, HAI, DAS, DTS), quality of life (WHO-Bref) and spirituality (FACIT). Measures are shown at 2 weeks post-dose 1 (psilocybin first n=14, niacin first n=14) and at 26 weeks post-dose 2 (psilocybin first n=11, niacin first n=12); asterisks indicate significance level of comparison to the niacin first group at 2 weeks post-dose 1. There were no significant differences between the psilocybin first group at 2 weeks post-dose 1 versus the psilocybin first group at 26 weeks post-dose 2.