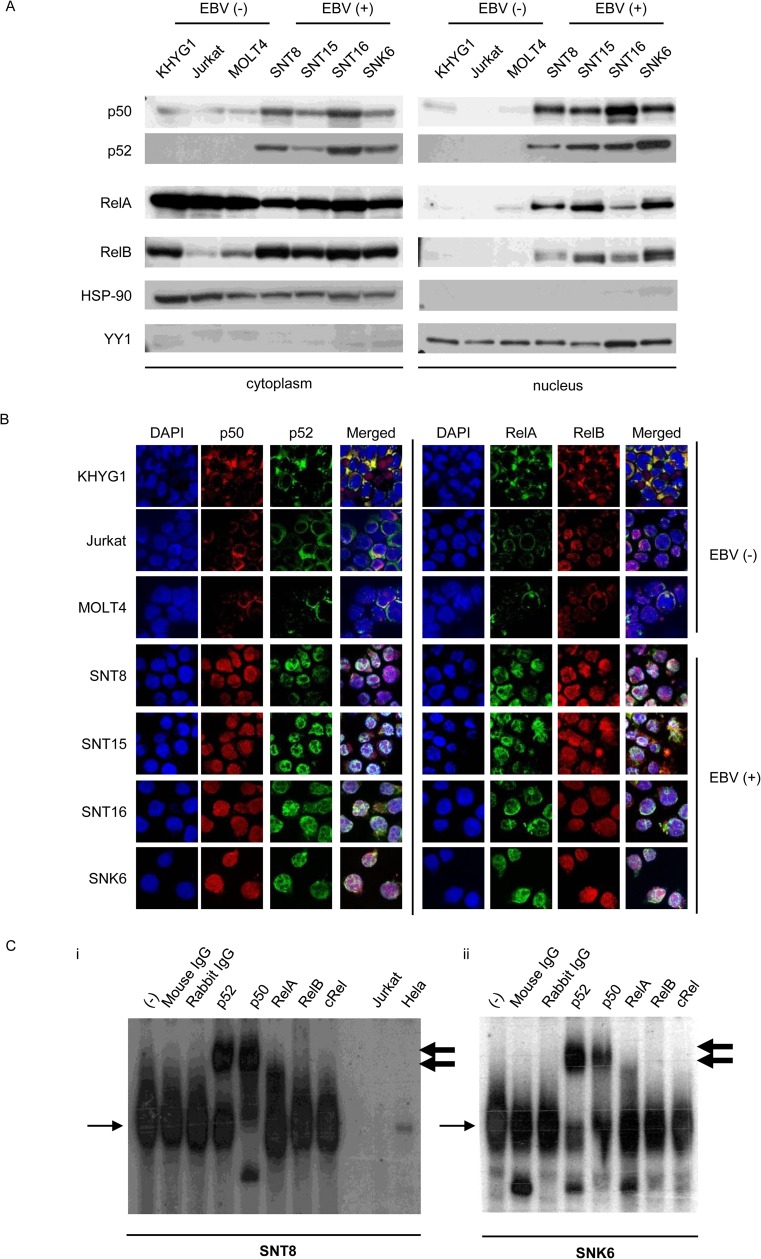
Fig 1. NF-κB activation in Epstein-Barr virus positive T or NK cells.
(A) Western blotting for NF-κB protein localization in Epstein-Barr virus (EBV)-positive T or NK cell (EBV-T/NK cell) lines. The same samples were electrophoresed twice and transferred onto two membranes: one membrane for anti-p50 staining, and the other for p52, RelA, and RelB antibody staining. HSP90 and YY1 were proteins that localized to the cytoplasm and nucleus, respectively. (B) Immunofluorescent staining for NF-κB protein localization in EBV-T/NK cell lines. Immunofluorescent staining with anti-p50, p52, RelA, and RelB antibodies was performed as indicated. DAPI was used for nuclear staining. The EBV-negative cell lines, KHYG1, Jurkat, and MOLT4, were used as negative controls. Cells were analyzed via confocal microscopy. (C) Electrophoretic mobility shift assay of SNT8 (i) and SNK6 (ii) cells. Nuclear extracts were obtained, incubated with a KBF-1 probe containing the NF-κB binding sequence with or without indicated antibodies, and subjected to the assay. The thin, black arrows indicate KBF-1-binding NF-κB proteins. The thick, black arrows indicate supershifted bands. Nuclear extracts of Jurkat cells and HeLa cells treated with 10 ng/ml of TNF-α for 30 min were used as controls.