Abstract
Hematopoietic stem and progenitor cells (HSPCs) egress from bone marrow during homeostasis and at increased rates during stress; however, the mechanisms regulating their trafficking remain incompletely understood. Here we describe a novel role for lipid receptor, sphingosine-1-phosphate receptor 3 (S1PR3), in HSPC residence within the bone marrow niche. HSPCs expressed increased levels of S1PR3 compared to differentiated bone marrow cells. Pharmacological antagonism or knockout of S1PR3 mobilized HSPCs into blood circulation, suggesting that S1PR3 influences niche localization. S1PR3 antagonism suppressed bone marrow and plasma SDF-1, enabling HSPCs to migrate towards S1P-rich plasma. Mobilization synergized with AMD3100-mediated antagonism of CXCR4, which tethers HSPCs in the niche, and recovered homing deficits of AMD3100-treated grafts. S1PR3 antagonism combined with AMD3100 improved re-engraftment and survival in lethally irradiated recipients. Our studies indicate that S1PR3 and CXCR4 signaling cooperate to maintain HSPCs within the niche under homeostasis. These results highlight an important role for S1PR3 in HSPC niche occupancy and trafficking that can be harnessed for both rapid clinical stem cell mobilization and re-engraftment strategies, as well as the opportunity to design novel therapeutics for control of recruitment, homing, and localization through bioactive lipid signaling.
Keywords: S1P3 receptor, hematopoietic progenitor cells, hematopoietic stem cell mobilization, stem cell niche, sphingosine-1-phosphate
Introduction
Hematopoietic stem and progenitor cells (HSPCs) are multipotent cells responsible for production of all blood lineages. HSPCs reside in specialized bone marrow (BM) niches; however, they are also able to traffic to blood and extramedullary sites when given appropriate cues. Dynamic control of HSPC localization is mediated through chemokines, cytokines, cell-cell, and -matrix interactions [1–8]. These trafficking control mechanisms can be utilized therapeutically to promote egress of HSPCs into blood, where the cells can be collected for transplantation into patients undergoing irradiation or chemotherapy. Peripheral blood HSPC grafts provide significant advantages over BM grafts, including superior re-engraftment with reduced hospital time, cost, and donor discomfort [9, 10]. Standard clinical mobilizing agents granulocyte-colony stimulating factor (G-CSF) and AMD3100 are successful in many patients, however these therapies are not universally effective and have limitations. G-CSF takes days to mobilize sufficient cell numbers for transplantation and causes significant complications under certain disease states such as sickle cell disease [11]. AMD3100 is a CXCR4 antagonist that blocks the interaction of CXCR4 with its ligand stromal-derived factor-1α (SDF-1), which plays a critical role in tethering HSPCs within the BM niche. AMD3100 induces rapid egress of HSPCs into blood, but impairs the cells’ ability to return to the niche since CXCR4 is necessary for homing and re-engraftment [12–14]. A deeper understanding of the innate mechanisms of HSPC niche occupancy, trafficking, and homing is critical to improve therapies for HSPC mobilization and re-engraftment post-transplantation.
Bioactive lipids, including sphingosine-1-phosphate (S1P), have emerged as additional endogenous control points in HSPC trafficking [15–18]. S1P is a chemoattractant for many immune cells, including HSPCs, and is maintained as a steep gradient between blood and BM [18]. High plasma S1P (around 1μM) sets up an antiparallel gradient with respect to high BM SDF-1 produced by stromal cells, which under steady state conditions localizes HSPCs within the SDF-1-rich BM [1, 19]. Loss of S1P gradients by inhibition of the S1P-degrading enzyme, S1P lyase, results in HSPC sequestration in the BM, suggesting the S1P gradient is necessary for HSPCs to leave the BM and enter peripheral blood. Plasma S1P is elevated during inflammation and in response to G-CSF and AMD3100 treatment, shifting the SDF-1/S1P gradient balance, which may be an important endogenous signal for trafficking of HSPCs and other immune cells [7, 15, 17, 20].
S1P is a ligand for five G-protein coupled receptors (GPCR), S1PR1-5, regulating processes including migration, matrix adhesion, and cell-cell contact, among other functions [21]. HSPCs express at least S1PR1-4 [8] and previous reports establish a role for S1PR1 in their trafficking to blood [15, 17]; however, the role of other S1PRs in HSPCs remains unclear. Multiple lines of evidence support the existence of cross-talk and/or synergy between the S1PR family and CXCR4/SDF-1 signaling axis. AMD3100-induced mobilization is abrogated in sphingosine kinase 1 (SPHK1) knockout mice, which have a substantially reduced plasma S1P concentration [15, 17, 22]. Agonism of S1PR1 by the selective small molecule SEW2871 boosts AMD3100-mediated mobilization but has no effect as a single agent, while functional antagonism of S1PR1 with FTY720 sequesters HSPCs in BM [15, 17]. While S1PR3 parallels many of the activities of S1PR1, S1PR3 is coupled to distinct signaling modules and may differentially regulate cellular processes. Monocytes expressing high S1PR3 exhibit enhanced migration toward SDF-1 upon S1PR3 stimulation, suggesting synergy of CXCR4 and S1PR3 [23]. S1PR3 is implicated in coordinating cross-talk of CXCR4-S1PR3 through S1P-mediated transactivation of the CXCR4 receptor [23, 24]. Similarly, S1PR3 engages in cross-talk with other growth factor receptors including platelet-derived growth factor receptor (PDGFR) by enhancing activation of intracellular signaling cascades such as Akt [25]. Taken together, there is significant evidence that S1PR signaling can modulate activity of cells through interaction with other receptor signaling axes, including CXCR4; however, the role of S1PR3 in HSPCs and the potential impact on CXCR4-regulated BM niche residence has not been explored.
The current study investigates the role of S1PR3 in supporting trafficking of HSPCs between BM and circulation and the relationship of S1PR3 signaling with the SDF-1/CXCR4 signaling axis. This work demonstrates that S1PR3 expression is higher in HSPCs than other BM cells types, allowing differential signaling by S1PR ligands in HSPCs compared to other cells. Acute antagonism or global knockout of S1PR3 promotes egress of HSPCs from BM to peripheral blood, which requires hematopoietic expression of S1PR3. The mobilization induced by blocking S1PR3 synergizes with CXCR4 antagonism. In contrast to CXCR4 antagonism, HSPCs mobilized via acute S1PR3 antagonism retain niche homing capacity in irradiated hosts. Synergistic antagonism of S1PR3 and CXCR4 improves homing, engraftment, and survival in irradiated hosts, indicating that CXCR4-dependent inhibition of HSPC homing and re-engraftment by AMD3100 can be functionally recovered through S1P signals. Further elucidation of lipid-based mechanisms of trafficking of HSPCs will allow the fine-tuning of strategies for directed control of the localization of endogenous stem cell populations.
Materials and Methods
Mice and in vivo assays
Animal studies were approved by the Institutional Animal Care and Use Committees at Georgia Institute of Technology or University of Virginia. C57BL/6J wildtype (WT), B6.SJL-PtprcaPepcb/BoyJ (CD45.1) (Jackson Laboratories) or S1PR3 knockout (KO) (MMRRC) mice were used for all studies (male, 8–12wks). Mobilization agents or vehicle were injected i.p. in PBS with 3% fatty acid free-BSA (FAF-BSA) or 2% hydoxypropyl-β-cyclodextrin at 5mg/kg. (VPC01091, Kevin Lynch, University of Virginia or Avanti Polar Lipids). G-CSF (125 μg/kg, s.c., Biolegend) was injected twice daily with analysis 3h after final injection. For chimera creation, mice were irradiated with 10.5 Gy (5.5-Gy/5-Gy doses, 3h apart) immediately prior to cell transplantation. Animals received sulfonamide or Baytril. Chimeras for mobilization (Figure 5) were created by injection of 8×106 hematopoietic cells per animal by jugular vein after lethal irradiation; animals were allowed 10 weeks to stabilize prior to mobilization. In acute homing assay: 3×106 CD45.1 BM cells were pre-treated with vehicle, 100nM VPC01091, 5μg/mL AMD3100, or both VPC01091+AMD3100. Drug-treated CD45.1 cells and competitive graft (1×106 untreated BM CD45.2 cells) were injected by jugular vein. In long-term engraftment studies, graft was comprised of blood-derived mononuclear cells from a fixed volume of donor blood after mobilization supplemented with 1×106 untreated BM CD45.1 cells. For graft threshold experiment (Figure 7E–F), supplemental graft was reduced to 1×104 BM cells.
Figure 5. S1PR3 on HSPCs is critical for mobilization.

(A) Schematic of irradiation chimera creation of CD45.1+ mice transplanted with donor (CD45.2) hematopoietic cells from WT or S1PR3 KO mice. (B) VPC01091-induced mobilization (1.5h) is maintained in WT but not S1PR3 KO chimeras. Data expressed as mean ± SEM. *p<0.05, n=4–5.
Figure 7. S1PR3 antagonism mobilizes functional HSPCs capable of re-engraftment in an irradiated host.

(A) Mobilized cell grafts were generated from peripheral blood of CD45.2 mice and combined with a competitive BM graft from CD45.1 mice. Chimeras were generated by transplanting grafts after lethal irradiation of CD45.1 hosts. (B–D) Peripheral blood was analyzed for chimerism at 6, 12, and 16 weeks using the CD45.2 allele (n= 4–6 animals) (E) Chimeras were generated using a supplemental graft of 10,000 BM cells to be at a threshold of successful graft to test the early graft survival advantage of VPC01091+AMD3100. Mobilized donor chimerism at 8wks demonstrates enhanced early chimerism with grafts mobilized by VPC01091+AMD3100 (n=3–6 mice per group). (F) Survival curve of lethally irradiated animals receiving peripheral blood transplants from AMD3100 or VPC01091+AMD3100 mobilized mice (n=8 mice per group). Data expressed as mean ± SEM (on logarithmic axis in B–D). *p<0.05.
Flow cytometry
Sample preparation was conducted as previously described using a BD FACSAriaIII and analyzed with FlowJo software [26].
Real-time PCR
Total RNA was isolated using RNeasy Mini or Micro Kit (Qiagen), reverse transcription by high-capacity cDNA kit (Applied Biosystems) with random primers, quantitative PCR was performed using SYBR Green Master Mix. Data normalized to GAPDH and quantified by the 2−ΔΔCT method. Primers: S1PR1: F: GGTGTAGACCCAGAGTCCT, R: GGTGTAGACCCAGAGTCCT; S1PR3: F: TCAACACTCTTCCCGCAGTC, R: CCCGGAGAGTGTCAT TTCCC; GAPDH: F: ACCACAGTCCATGCCATCAC, R: TCCACCACCCTGTTGCTGTA; SDF-1: F: CACAGTTTGGAGTGTTGAGGA, R: CGCTCTGCATCAGTGACG; SPHK1: F: GATCCCTTGGAGTCGGTGTC, R: TCTTATCGGTGTTGCCCAGG.
Colony-forming assay
Peripheral blood or sorted cells were plated in Methocult™ GF-M3434 (Stemcell Technologies). Samples were plated in triplicate and colonies were counted 6 or 12d after plating.
Migration assays
WBM cells or sorted BM LSK were placed in a 5μm porous membrane Boyden transwell chamber (96-well format, 0.5–1×105 cells; 24-well format, 2–5×105 cells) containing IMDM + 0.5% FAF-BSA with or without a competing murine SDF-1α signal (Peprotech) and migrated for 2–4h toward vehicle, S1P, SDF-1α, or mouse plasma. In Figure 2C, no chemotactic agent was added to the bottom of the transwell chamber to allow observation of spontaneous migration in either media (Control) or VPC01091-treated conditions.
Figure 2. HSPCs utilize S1PR3 for migration and adhesion.

(A) S1P and SDF-1 induce migration of sorted LSK cells in a 2hr transwell migration assay (n=3 independent experiments). (B) Antagonism of S1PR3 with VPC01091 dose-dependently reduces acute adhesion of Linneg-enriched cells to a fibronectin-coated surface (n= 3 independent experiments). (C) S1PR3 antagonism by VPC01091 (100 nM) increases the general motility of WBM in a transwell chamber without chemoattractant (n=4 independent experiments with 3–4 replicates per group). (D) VPC01091 suppresses ERK phosphorylation compared to baseline in LSK cells sorted from WBM, assayed using “in-cell western” (Cell Signaling Technologies) (n=5 independent). Data expressed as mean ± SEM. *p<0.05.
Plasma charcoal stripping
Activated charcoal (4g/L) was incubated with plasma with rotation, 4°C overnight. Charcoal was pelleted by centrifugation 1000×g 10min and supernatant was sterile filtered. Charcoal-stripped plasma was stored at −80°C until use.
Adhesion assays
WBM was enriched for HSPCs using EasySep™ Mouse Hematopoietic Progenitor Cell Enrichment Kit (Stemcell Technologies) as described by the manufacturer. Adhesion assay was adapted from [27]. Fibronectin-coated plates (10μg/mL) were blocked 1h with 5% FAF-BSA/PBS. HSPCs were plated into the coated 96-well plate with vehicle or VPC01091. Cells were centrifuged to the bottom of the plate for 15s and allowed to adhere at 37°C for either 2 or 30 min. Non-adherent cells were removed by three gentle PBS washes and stained with DRAQ5 (1:5000) prior to imaging by Licor Odyssey. Relative cell adhesion was quantified by relative total fluorescence intensity.
SDF-1 and sphingolipid analysis
For plasma analysis blood was centrifuged 10min, 1000×g; supernatant was spun at 1000×g, 10min and then stored at −80°C. Sphingolipid extraction and quantification were performed as previously described [20, 28]. Measurement of SDF-1 was performed using the Mouse SDF-1alpha Quantikine ELISA Kit (R&D Systems) as described by the manufacturer, which detects amino acids 22–89 of SDF-1. BM protein was collected by suspending BM cells in PBS (50μL), centrifuging, and collecting the supernatant. Protease inhibitors were added and protein was stored at −80°C until analysis.
OP9 stromal cell culture and staining
OP9 cells were cultured in 0.5% Puramatrix (BD Biosciences) as previously described [29]. Cells were fixed, permeabilized, blocked and incubated with anti-mouse SDF-1α (R&D Systems). Fluorescent secondary antibody was used for visualization with DAPI and fluorescently tagged phalloidan (Life Technologies) to visualize nuclei and filamentous actin.
Intracellular signaling assays
BM cells were isolated from WT or S1PR3 KO animals. Cells were serum starved in IMDM at 37°C for 3hrs and then treated for 15 mins with 200nM SDF-1α (Peprotech) or vehicle in serum-free IMDM. Cells were pelleted and processed according to manufacturer’s protocol for PathScan RTK Signaling Antibody Array Kit (Cell Signaling Technology). Slide arrays were imaged and quantified on Licor Odessey. Signal intensity was normalized to WT control and presented as fold change. Data is from two independent experiments run in duplicate. For ERK1/2 analysis, LSK cells were sorted by FACS and treated with 100 nM VPC01091 or vehicle control. At the indicated time points, cells were fixed and analyzed using PhosphoPlus® p44/42 MAPK In-Cell Duet (Cell Signaling) as described by the manufacturer.
Statistical analysis
Data are presented as mean ± standard error. Multiple comparisons were performed by one- or two-way ANOVA followed by Dunnett or Sidak post-tests to compare means, or Kruskal-Wallis test for non-parametric comparisons. Two-group comparisons used unpaired, 2-sided t-test. Survival analysis by log-rank (Mantel-Cox) test with a chi square distribution. All statistical analysis used GraphPad-Prism software; p<0.05 was considered significant.
Results
S1PRs are differentially expressed within the BM niche
To gain insight into how S1PR signals play a role within the HSPC niche, we performed analysis of mRNA in sorted BM populations (Figure 1A–B). Differential expression of S1pr1 and 3 was observed, suggesting distinct roles for S1P signaling within different BM populations. S1pr1 expression was similar among lineage-committed (Lin+), non-lineage-committed (Linneg) cells, and HSPCs (Linneg Sca-1+c-Kit+ (LSK)); however, mesenchymal stromal cells (MSCs) (CD29+CD44+CD90+Sca-1+) expressed significantly higher levels of S1pr1 mRNA (Figure 1A). Interestingly, S1pr3 mRNA is nearly 2.5-fold higher in the LSK population than Lin+, Linneg, or MSCs (Figure 1B). Surface expression of S1PR3 assessed by flow cytometry was higher on LSK cells compared to whole bone marrow (WBM) or Lin+ cells (Figure 1C–D). To test whether S1PR3 expression is a hallmark of hematopoietic progenitors, BM cells were sorted based on their S1PR3 expresssion level and plated into an in vitro colony forming unit (CFU) assay. S1PR3+ sorted BM cells had higher CFU activity than S1PR3neg/low cells, indicating the presence of more progenitors within the S1PR3+ fraction (Figure 1E). S1PR3 expression level on sorted cells was validated by western blotting (Figure S1). Distinct S1P receptor expression profiles may allow constituents of the BM niche to tailor unique, cell-type specific responses to S1P; therefore, higher expression of S1PR3 on HSPCs could play an important role in regulating progenitor cell activity, including niche residence and migration.
Figure 1. S1PRs are differentially expressed within the BM microenvironment.
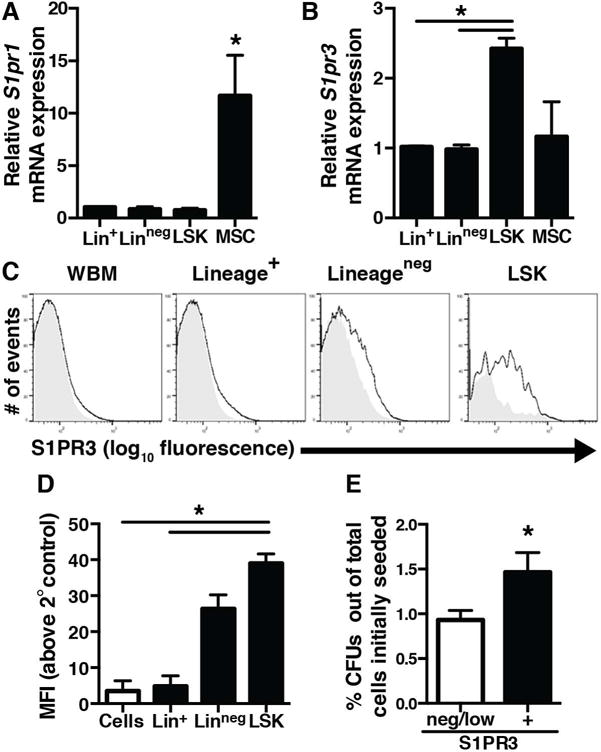
(A) S1pr1 mRNA expression in lineage-committed (Lin+), non-committed (Linneg), HSPCs (LSK), and MSCs (CD29+CD44+CD90+Sca-1+) (n=3–4 independent experiments). (B) S1pr3 mRNA expression is higher in LSK progenitor population (n=3–4 independent experiments). (C) Cell surface S1PR3 expression in murine WBM by flow cytometry. Shaded histograms are the indicated population stained without the S1PR3 primary antibody to demonstrate background fluorescence (n=3 mice). (D) Median fluorescence intensity (MFI) for S1PR3. (E) The S1PR3+ cell population sorted from BM is enriched for CFU activity relative to a S1PR3neg/low population (n=3 independent experiments with 2–3 replicates per group). Data expressed as mean ± SEM. *p<0.05.
HSPCs functionally respond to S1PR ligands
Although HSPCs express many chemotactic receptors, they are selective in the signals to which they functionally respond [30]. S1PRs regulate a number of cellular functions, including migration and adhesion [31, 32]. Sorted HSPCs migrate in a dose-dependent manner towards S1P in vitro, indicating that HSPCs detect and initiate chemotaxis toward S1P gradients (Figure 2A), consistent with previous reports [18]. VPC01091, a selective S1PR3 antagonist [33], rapidly decreases the ability of Linneg-enriched cells to adhere to fibronectin, suggesting the S1PR axis is important for acute regulation of cell-matrix interactions (Figure 2B). Further, VPC01091 enhanced general motility of BM cells without chemoattractant (Figure 2C), indicating that S1PR3 signaling may regulate hematopoietic cell motility. S1PR3 signaling is coupled to G-αi and can signal through ERK activation [34]. Antagonism of S1PR3 in LSK cells decreases p-ERK relative to total ERK, suggesting that these progenitors have basal S1PR3-mediated downstream signaling that can be pharmacologically modulated (Figure 2D).
Antagonism of S1PR3 stimulates acute trafficking of HSPCs
Given the differential expression of S1PR3 on HSPCs, the current study sought to investigate whether S1PR3 plays a role in regulating egress of HSPCs out of the BM niche. Animals were treated with the S1PR3 antagonist VPC01091, to transiently block S1PR3 signaling [33]. VPC01091 treatment induced a 2-fold increase in circulating number of HSPCs (identified as LSK) within 1.5hrs (Figure 3A–B), indicating that acute interference with S1PR3 signaling liberates HSPCs into circulation. The selective CXCR4 antagonist AMD3100, a clinical mobilizing agent, similarly induces increased circulation of HSPCs within 1h of treatment. To test the interplay of S1PR3 with CXCR4 signaling in HSPC mobilization, we pre-treated animals with VPC01091 and then delivered AMD3100 or vehicle control. S1PR3 antagonism enhanced AMD3100-mediated mobilization (Figure 3A), suggesting that S1PR3 may play a role in maintaining HSPC niche residence. Mobilized HSPCs demonstrated functional ability to form colonies in a CFU assay (Figure 3C). In support of the role of S1PR3 in maintaining HSPCs in the niche, genetic knockout of S1PR3 leads to higher homeostatic circulation of HSPCs and enhanced mobilization in response AMD3100 (Figure 3D). VPC01091 mobilization was transient, returning to baseline by 24h (Figure 3E), and produced an equivalent increase in circulating LSK cells in 1.5h as G-CSF administration for 2d (the earliest timepoint of HSPC mobilization in a G-CSF regimen [35]), indicating that selective modulation of S1PR signaling provides a rapid strategy for HSPC mobilization (Figure 3F).
Figure 3. S1PR3 antagonism stimulates acute and selective trafficking of HSPCs.

(A) Mice were injected with saline vehicle (Cont), AMD3100 (AMD), VPC01091 (VPC), or VPC01091+AMD3100 (V+A). Circulating LSK were quantified in peripheral blood after 1.5h (n>3 mice per group). (B) Representative flow cytometry plots for blood LSK analysis. (C) CFU in peripheral blood 1.5h after treatment (n=4–7 mice per group). (D) S1PR3 KO mice have more circulating CFU which is enhanced by AMD3100 (n=6–7 mice per group). (E) WT BM cells induce the phosphorylation of Akt in the presence of SDF-1 while S1PR3 KO BM cells have reduced Akt phosphorylation. Data expressed as mean ± SEM. *p<0.05. (F) VPC01091-induced mobilization of LSK cells is transient and returns to steady state by 24h after injection (n=4 mice per group). (G) VPC01091 (1.5h, 5 mg/kg) mobilizes a similar number of LSK cells as early G-CSF (2 days, 250 μg/day) (n=4 mice per group).
Previous reports have suggested that SDF-1/CXCR4-stimulated activity of Akt in HSPCs is critical to their ability to adhere to the BM stromal compartment and depression of Akt signaling promotes migratory behavior of HSPCs [36]. S1PR3 is also required for the activation of Akt [25]. While SDF-1 increases p-Akt (Ser473) in WT BM cells in vitro, loss of S1PR3 in KO cells leads to a decrease in SDF-1-stimulated Akt phosphorylation (Figure 3G). These data suggest that the S1PR3 axis interacts with the SDF-1/CXCR4 axis through potentiation of common intracellular signaling cascades to promote adhesion to the BM niche [36]. Consistent with this finding, antagonism of S1PR3 reduced the ability of HSPCs to adhere to fibronectin matrix (Figure 2B) and increased motility (Figure 2C).
To further investigate the relative roles of S1PR1 and 3 in acute mobilization, additional pharmacological agents were tested for mobilization activity (Figure S2A). VPC01091 is a weak partial agonist at S1PR1, meaning it has a similar EC50 to the native ligand S1P; however, it has less than half the efficacy of recruiting membrane bound GTP making it classified as a weak partial agonist [33]. SEW2871, a full agonist of S1PR1 [37], did not induce mobilization (Figure S2B). SEW2871 has been previously shown to enhance mobilization when combined with CXCR4 antagonism, however S1PR1 agonism alone is not sufficient for egress [17]. The S1PR1 functional antagonist FTY720 and S1PR1 antagonist W146 did not enhance circulating LSK cells after 1.5h (Figure S2B) [15]. S1PR3 antagonism also altered acute trafficking of lymphocytes and neutrophils that was not observed with S1PR1 agonism alone, suggesting distinct effects of each receptor on hematopoietic cell populations (Figure S2C). Taken together, although VPC01091 has weak activity at S1PR1, when considered with the evidence from the S1PR3 genetic KO and the lack of mobilization with SEW2871, our data support a role for S1PR3 in negatively regulating egress of HSPCs from the niche.
SDF-1 regulates S1P-mediated chemotaxis of HSPCs
In addition to cell-intrinsic signaling, shifting chemotactic gradients between the BM and blood are implicated in controlling trafficking of HSPCs during stress and in response to mobilizing agents [15, 17, 18]. Sorted BM LSK cells exhibited enhanced migration toward plasma from VPC01091-treated animals in vitro compared to control plasma (Figure 4A). Plasma lipid removal by charcoal stripping abrogated this effect, indicating that lipid species in part mediate the increased chemotactic potential of plasma (Figure 4A). Plasma S1P and sphingosine concentrations did not significantly change (Figure 4B–C). S1P was undetectable in BM extracellular fluid, and BM Sphk1 mRNA did not change (Figure S3A). Given that no changes in S1P were observed, we hypothesized that S1PR3 signaling may alter SDF-1 plasma levels, which provides a competing signal to S1P. Total plasma SDF-1 decreased transiently and normalized by 24h with VPC01091 treatment (Figure 4D), which negatively correlates with HSPC mobilization (Figure 3E). Endothelial cells treated with VPC01091 reduced secretion of SDF-1 over 1.5h in vitro (Figure 4E). VPC01091 also decreased total extracellular SDF-1 in BM (Figure 4F) but did not change Sdf1 mRNA expression or SDF-1 intracellular protein levels (Figure S3B–C). VPC01091 also decreased SDF-1 secretion in vitro from OP9 murine BM stromal cell line (Figure 4G) [29]. Conversely, FTY720 (S1PR3 agonist, S1PR1 functional antagonist) increased secretion of SDF-1 by OP9 BM stromal cells (Figure S3D–E), further supporting the hypothesis that S1PR signaling modulates SDF-1 secretion. To examine how anti-parallel gradients of SDF-1 and S1P affect BM cell migration, we developed an in vitro competitive gradient migration assay (Figure 4H). The presence of SDF-1 (L-SDF: 1.25nM, H-SDF: 12.5 nM) in the top of the Boyden chamber abrogated migration of BM cells toward S1P (1μM) (Figure 4H), further indicating that SDF-1 and S1P gradients compete for BM cell attraction. Taken together, these results support the hypothesis that VPC01091 reduces systemic SDF-1 levels, enabling lipid-mediated entrance of HSPCs into circulation.
Figure 4. SDF-1 inhibits S1P-mediated migration.

(A) Plasma from VPC01091-treated animals is more chemotactic for LSK cells than control plasma in a transwell migration assay, which is abrogated by charcoal stripping (n>5 per group, 2–3 independent experiments). HPLC mass spectrometry analysis of plasma (B) S1P and (C) sphingosine 1.5h after VPC01091 (n=17–18 mice per group). (D) Plasma SDF-1 level transiently decreases with VPC01091 treatment (n>3mice per group). (E) Endothelial cell SDF-1 secretion 1.5h after VPC01091 (1 μM) treatment in vitro (n=3 replicates per group). (F) BM extracellular SDF-1 1.5h after VPC01091 treatment (n=14–16 mice/group, 3 independent experiments). (G) Treatment of OP9 MSCs with VPC01091 (n=2 independent experiments, 3–4 replicates per group). (H) SDF-1 (L=1.25nM, H=12.5nM) inhibits migration of WBM to S1P (0.5uM) in a transwell migration assay (n=3 independent experiments with 3–4 replicates per group). Data expressed as mean ± SEM. *p<0.05.
S1PR3 on HSPCs is critical for mobilization
To explore the relative contribution of S1PR3 signaling on HSPCs versus S1PR3 in the niche environment, we generated irradiation chimeras of CD45.1+ mice transplanted with donor hematopoietic cells from WT or S1PR3 KO mice (Figure 5A). Donor cells were tracked by the CD45.2 allele. Since MSCs are irradiation resistant at doses that induce HSC apoptosis [38], the WT BM microenvironment can be maintained during total body irradiation [39]. S1PR3 KO chimeras showed an increased trend in circulating HSPCs after 10 weeks, suggesting that loss of hematopoietic S1PR3 promotes enhanced HSPC circulation (p=0.07). VPC01091-induced mobilization in the WT chimera but not in the KO chimera (Figure 5B), suggesting that S1PR3 on hematopoietic cells is necessary for mobilization of HSPCs. While not significant, the KO chimeras displayed a trend of increased circulating LSK cells in response to VPC01091 (p=0.33) supporting the notion that both niche and HSPC-intrinsic S1PR3 signaling may contribute to robust mobilization.
Acute S1PR3 antagonism maintains the homing potential of HSPCs
Acute homing of HSPCs to the BM niche is necessary for survival and long-term re-engraftment and can be significantly impaired by treatment with clinically-used mobilization therapies [13]. To investigate the homing capacity of cells exposed to VPC01091, we treated CD45.1+ WBM grafts ex vivo with VPC01091, AMD3100, or VPC01091+AMD3100. The pre-treated grafts (CD45.1) and an untreated competitive graft of CD45.2 WT BM (3×106 cells:1×106 cells, respectively) were transplanted immediately after lethal irradiation and acute BM homing was assessed 24h later (Figure 6A). AMD3100-treated LSK cells had reduced homing capacity, while VPC01091 grafts displayed no deficits in acute homing (Figure 6B). Further, VPC01091 pre-treatment with AMD3100 rescued the homing capacity of AMD3100-treated cells, however the mechanism is unclear.
Figure 6. S1PR3 antagonism does not inhibit HSPC BM homing.

(A) The effect of S1PR and CXCR4 signals on in vivo HSPC homing was assessed in an acute (24h) competitive homing assay. CD45.1 BM cells were pre-treated ex vivo with groups indicated. Cells were transferred to lethally irradiated WT mice and BM chimerism was analyzed 24h later. (B) BM chimerism analysis for CD45.1 donor marker in the LSK population. AMD3100 blocks acute homing of LSK cells, while VPC01091 does not (n>3 mice per group, 2 independent studies). (C) Frequency of CXCR4+ LSK cells in circulation and (D) the MFI of CXCR4 on LSK cells after VPC01091 (n=4 mice per group). Data expressed as mean ± SEM. *p<0.05.
In mobilized blood, there were more CXCR4+ HSPCs in circulation 1.5h after VPC01091 treatment compared to control (Figure 6C, D). CXCR4 expression on mobilized HSPCs may enable cells to return to the BM after transplantation, as SDF-1 is an important HSPC homing signal and is upregulated in irradiated BM [40]. These data indicate that S1PR3 antagonism enables HSPC egress from the BM without impairment of homing to SDF-1-rich domains compared to AMD3100-treated HSPCs.
S1PR signaling enhances early engraftment of AMD3100 HSPC grafts
To examine the re-engraftment potential of cells mobilized by S1PR3 antagonism, CD45.2 mice were mobilized with either AMD3100, VPC01091, or VPC01091+AMD3100. An equal volume of peripheral blood was collected and cells were supplemented with a competitive BM graft (1×106 CD45.1 cells) prior to transplantion into lethally irradiated CD45.1 mice (Figure 7A). Blood was assayed at 6, 12, and 16wks for the presence of CD45.2+ cells derived from the mobilized graft. At the early timepoint of 6 weeks, donor chimerism parallels the mobilization response observed in immunophenotyping and CFU assays (Figure 7B, 3A–C), suggesting that mobilized HSPCs re-engraft in the irradiated host and give rise to progeny in vivo. At longer timepoints, the AMD3100-treated groups maintained peripheral repopulation, however the VPC01091-mobilized graft did not (Figure 7C–D). To determine whether the addition of VPC01091 to AMD3100 therapy enhanced overall engraftment and survival of HSPC grafts, an additional chimerism experiment was conducted where the supplemental BM graft was selected below a threshold of cells required for graft success [41]. At 8wks, more donor cells were found in the blood of VPC01091+AMD3100-grafted animals compared to AMD3100, supporting the S1PR3 and CXCR4 synergy (Figure 7E). VPC01091+AMD3100 grafts supported higher survival rate than AMD3100, suggesting that adjunct VPC01091 therapy mobilizes enough additional HSPCs for successful short-term repopulation of the hematopoietic system, particularly enhancing early survival and repopulation capacity (Figure 7E–F). These findings suggest that antagonism of the S1PR3 and CXCR4 signaling axes coordinates to rapidly mobilizes stem and progenitor cell population and therefore both receptor signals are important for HSPC niche maintenance.
Discussion
Lipid-based signaling mechanisms controlling the trafficking of HSPCs remain incompletely understood. The current study finds that activity of S1PR3 plays a role in determining HSPC niche occupancy or egress, suggesting that S1PR3 works opposite S1PR1 and in synergy with CXCR4 in controlling stem and progenitor cell localization. Antagonism of S1PR3 signaling affects both HSPC cell-intrinsic signaling and niche cues to contribute to mobilization. These results present an additional level of control in the complex signaling modules governing HSPC trafficking and identify sphingolipids and their receptors, particularly S1PR3, as important regulators of HSPC localization.
Despite the low interstitial BM concentration of S1P, the majority of BM cells express S1PRs. Relative S1PR expression profiles contribute to the spectrum of cellular responses to S1P. For example, expression of S1PR1 and S1PR2 in hematopoietic osteoclast precursors counterbalances positive and negative migratory response to S1P gradients [42]. HSPCs express nearly 2.5-fold more S1pr3 mRNA than other BM-derived cells (Figure 1B). In sub-populations of human HSPCs, more primitive CD34+/CD38− cells express S1PR3, but is undetectable in CD34+/CD38+ HSPCs [43]. Prior literature reports that S1P has a higher binding affinity for S1PR3 than S1PR1, therefore S1PR3 signaling may dominate in HSPCs in the BM where S1P levels are low [44]. The prevalence of S1PR3 on HSPCs relative to other BM cells provides a unique S1P signaling signature and a novel target to selectively influence their trafficking.
GPCR modular signaling complexity underlies the receptor-specific response to S1P ligands. S1PR1 and 3 couple to G-αi, stimulating Rac, actin stress fiber formation, focal adhesions, integrin clustering, and ERK activation [31]. In addition to G-αi, S1PR3 also couples to G-α12/13, and G-αq. S1PR3 is implicated in Akt and MAP kinase signaling pathways including ERK1/2 [25, 45]. These signaling pathways contribute to adhesion of HSPCs to niche matrix and stromal cells. VPC01091 decreased adhesion and increased cell motility and chemotaxis in vitro (Figure 2). ERK activation can stimulate activity of SPHK1, thus causing a feed-forward autocrine loop by production of S1P ligand [46], thereby explaining the homeostatic ERK activity that is reduced by VPC01091 (Figure 2D). VPC01091 is a robust antagonist at S1PR3 and a weak partial agonist of S1PR1 with a much lower efficacy than S1P [33]. S1PR3 genetic KO phenocopies the mobilization of HSPCs observed with VPC01091 (Figure 3D), suggesting that S1PR3 rather than S1PR1 is a critical signal in niche localization of HSPCs. In mixed chimeras with S1PR3 KO hematopoietic cells and WT niche environment, S1PR3 cells were found at higher circulating rates at baseline compared to WT (p=0.07) (Figure 5B), suggesting this effect is partially cell-intrinsic. S1PR3 blockade may also sensitize cells to S1PR1-dominated responses such as migration and egress out of the BM. Hematopoietic conditional knockout or functional antagonism of S1PR1 reduces the effect of both G-CSF- and AMD3100-mediated mobilization [15, 17]. Despite a clear role for S1PR1 in HSPC egress, SEW2871 alone is insufficient to mobilize HSPCs (Figure S2B), likely due to competition with dominating niche retention signals through S1PR3 and CXCR4. Upon disruption of the CXCR4-SDF-1 tethering signal with AMD3100, SEW2871 can successfully mobilize [17], indicating the S1PR1 axis promotes BM egress (Figure S4). Consequently, HSPCs likely integrate distinct signals from S1PR1, S1PR3, and CXCR4 to make decisions about migration from the niche.
Previously, we have shown that S1PR3 agonism enhances chemotaxis toward SDF-1 in monocytes expressing high S1PR3 and the effect is absent in S1PR3 KO monocytes, suggesting S1PR3 enhances CXCR4-mediated activity [23]. S1PR3 in BM cells regulates CXCR4-mediated Akt phosphorylation (Figure 3G, S4), which is critical for adhesion to BM stromal cells [36], suggesting that S1PR3 tunes CXCR4-mediated responses. Mechanisms for such intracellular cross-talk are complex, however, may occur through receptor transactivation [24, 25]. In endothelial progenitor cells, S1P transactivates CXCR4 by Src-mediated tyrosine phosphorylation in an S1PR3-dependent manner [24]. Additionally, S1PR3 transactivates PDGFR to contribute to synergistic Akt activation [25]. Both S1PR3 and CXCR4 have been found in lipid raft fractions [47, 48]; therefore, it is possible that cross-talk between these receptors [24] occurs in lipid raft microdomains. For optimal SDF-1 signaling, CXCR4 must be localized to lipid rafts [49]. Disruption of lipid raft formation in human peripheral blood CD34+ cells impairs SDF-1-mediated migration [47]. Homeostatically, S1PR3 may sensitize HSPCs to SDF-1 by amplifying intracellular signals, while antagonism of S1PR3 transiently reduces HSPC sensitivity to SDF-1 gradients (Figure S4). Since S1PR3 antagonism only reduces rather than completely blocks CXCR4 sensitivity, the combined use of both VPC01091 and AMD3100 releases more HSPCs than either agent alone.
The lipid-chemokine balance between the niche and blood are crucial for migration. S1P is a major chemoattractant in the plasma that migration of hematopoietic lineage cells between BM, lymphoid organs, and blood depends on [15, 17, 22]. S1P may also be an endogenous mobilization signal during stress, as plasma S1P is elevated during acute inflammatory responses [20] and BM S1P increases following total body irradiation [50]. No change in S1P or sphingosine was detected in plasma 1.5h after VPC01091 administration (Figure 4B–C); however, lipid removal decreased the chemotactic potential of the plasma, suggesting that lipid species are a central chemotactic factor in VPC01091-mediated BM egress, likely through S1PR1 (Figure 4A). Additional lipid species may also play a role, as ceramide-1-phosphate has been shown to stimulate chemotaxis of HSPCs and other progenitor cells [51]. Our data suggest that lipids are necessary for HSPC chemotaxis toward plasma, but not a sustained change in S1P concentration. S1PRs regulate the production and secretion of cytokines and growth factors, including SDF-1 from BM niche cells [15, 23]. S1PR3 antagonism caused a decrease in BM SDF-1 protein secretion and in vitro OP9 MSC secretion (Figure 4, S3). Therefore, S1PR signaling may alter HSPC trafficking in part through modulating local SDF-1 production. BM cells failed to migrate towards S1P in the presence of a large competing SDF-1 gradient; however, reduction of SDF-1 enabled S1P-mediated migration (Figure 4H). Taken together, VPC01091 reduces the SDF-1 gradient between blood and BM (Figure 4, S3), enabling HSPCs to enter peripheral blood in a lipid-dependent manner.
Antagonism of CXCR4 poses a challenge for HSPCs returning to the niche due to its role in homing [52]. G-CSF+AMD3100 grafts fail to engraft with the same efficiency as G-CSF-mobilized cells alone, suggesting that AMD3100 impairs engraftment [13]. In our study, ex vivo CXCR4 antagonism reduced the ability of HSPCs to acutely home to irradiated BM in 24hrs, while homing was rescued by pre-treatment with VPC01091 (Figure 6C). Since BM SDF-1 and S1P are both elevated after irradiation, the increase in homing may reflect enhanced SDF-1 or S1P sensitivity in ex vivo treated cells [15, 50]. In mobilization studies, VPC01091 increased circulating CXCR4-expressing HSPCs, which could reflect egress of a CXCR4-hi population of LSK cells. Neutrophils dynamically up-regulate CXCR4 expression with time once they enter circulation in order to facilitate their return to the BM [53]. Moreover, HSPCs in circulation have higher surface expression of CXCR4 compared to BM resident HSPCs [54]. We cannot exclude the possibility that in VPC01091-treated animals, the HSPCs have been in circulation longer than HSPCs in control animals due to the transient loss of niche retention signals and therefore have increased expression of CXCR4.
Following transplantation into lethally irradiated mice, VPC01091-mobilized grafts enhanced short-term hematopoietic reconstitution, but were unable to support long-term repopulation (Figure 7B). Short-term HSPCs and more differentiated progenitors can become highly proliferative and perform emergency myelopoiesis during stress, either in their BM niches or in peripheral tissues [2, 8, 55]. While long-term HSPCs are both required and sufficient for permanent hematopoietic reconstitution, short-term HSPC populations may enable rapid reconstitution of myeloerythroid lineages and prevent the life-threatening cytopenia induced by myeloablation [56, 57]. Our studies suggest that combined S1PR3 and CXCR4 antagonism produced mobilized cell grafts that better facilitated early survival of irradiated hosts when the supplemental graft is near a limiting dose for survival (Figure 7E–F).
Understanding cues that regulate the physiological niche has broad implications for novel therapies. The gold standard for clinical isolation of blood-derived HSPCs for transplantation is G-CSF, yet it takes days to collect enough cells, is associated with bone pain, spleen enlargement, and increased risk of thrombosis, and is not successful in all patients [9]. VPC01091 mobilization rapidly increases the frequency of circulating progenitors (Figure 3), reduces graft lymphocyte content (Figure S2C) [33], is not associated with systemic inflammation (Figure S2C), and synergy with CXCR4 signaling provides opportunities to fine tune mobilization strategies. Control of niche occupancy may also be important for targeting pathological niche environments that protect malignant leukemic cells from irradiation and chemotherapy [59, 60]. In addition, evidence is mounting for HSPC roles in extramedullary sites, including homeostatic immunosurveillance, localized hematopoiesis at sites of injury, and potential contribution to regenerative processes [2, 8]. Regenerative medicine therapies that leverage mobilized or transplanted hematopoietic progenitors for extramedullary myelopoiesis are a promising strategy to complement conventional mechanisms of myeloid cell recruitment for repair of damaged tissues [2, 58]. Elucidation of endogenous mechanisms of stem cell migration presents opportunities to design novel therapeutics for control of recruitment, homing, and localization through S1P signaling.
Supplementary Material
(A) During homeostasis, high local SDF-1 concentrations signals through CXCR4 to phosphorylate Akt and promote HSPC retention within the niche through increased adhesion. Low interstitial S1P concentration supports activation of Akt through S1PR3. (B) Antagonism of CXCR4 by AMD3100 reduces SDF-1-mediated retention of HSPCs within BM. AMD3100 also increases plasma S1P concentration enabling HSPCs to migrate towards S1P-rich plasma using S1PR1. Lower Akt activation supports egress rather than retention. (C) Antagonism of S1PR3 reduces local SDF-1 concentrations, decreasing signaling through CXCR4, and reduces total Akt phosphorylation, allowing HSPCs to follow S1P signals to the plasma through S1PR1. (D) In the presence of VPC01091 and AMD3100, both S1PR3- and CXCR4-mediated retention within the BM niche are blocked and S1PR1-mediated signaling promotes maximal egress.
Cells sorted as S1PR3 high and low populations were digested and analyzed by western blot for expression of S1PR3 protein. High and low populations produced the expected band intensity with an independent S1PR3 antibody. Whole bone marrow (WBM) was used as control.
(A, B) Mice treated with selective agonists/antagonists of S1PR1 and S1PR3 signaling demonstrate differential mobilization of LSK cells into peripheral blood after 1.5h (n>3 mice per group). (C) S1PR3 antagonism by VPC01091, but not S1PR1 agonism by SEW2871, alters the trafficking of white blood cells, including neutrophils and lymphocytes (n>4 mice per group). Data expressed as mean ± SEM. *p<0.05.
(A) BM expression of Sphk1, (B) SDF-1 intracellular BM protein, and (C) Sdf1 mRNA in the BM of mice 1.5h after VPC01091 treatment (n=15–16 mice). (D) Secretion of SDF-1 by OP9 MSCs 1.5h after FTY720 treatment as detected by ELISA (n=2–3 independent experiments). (E) SDF-1 staining (red) of OP9 MSC in 3D culture 1.5h after FTY720 treatment (1 μM) shows a decrease in cellular SDF-1 and increase in diffuse SDF-1 signal after FTY720 treatment indicating secretion of the protein. (Nuclei, blue; scale bar 50um)
Acknowledgments
We thank Kevin Lynch (University of Virginia) for providing VPC01091 and advice on S1P receptor pharmacology. Richard Proia (NIH) for providing the S1PR3−/− mice; Cheryl Lau and Caitlin Powell for review of the manuscript; and the core facilities staff of the Parker H. Petit Institute for Bioengineering and Bioscience for technical expertise. Study supported by NIH Grants K01AR052352, R01AR056445, and R01DE019935, Regenerative Engineering and Medicine Center’s “Georgia Partners in Regenerative Medicine” grant to Dr. Botchwey, and the National Science Foundation grant NSF GRFP DGE-1148903 to Claire Segar.
Footnotes
Author Contributions:
Molly E. Ogle, Claire E. Segar, Anthony Awojoodu, Anusuya Das: Conception and design, collection/assembly of data, data analysis, manuscript writing, final approval of manuscript. Rafael A. Ortiz, Hoi Yin Cheung: Collection/assembly of data, data analysis, manuscript writing; Edward A Botchwey: Conception and design, financial support, final approval of manuscript.
Conflict of interest disclosure: E.A.B, A.O.A, A.D. disclose a potential conflict of interest relating to intellectual property in a pending patent application. The authors have no additional financial interests. M.E.O., C.E.S., R.A.O., H.Y.C. declare no conflict of interest.
References
- 1.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Si Y, Tsou CL, Croft K, et al. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest. 2010;120:1192–1203. doi: 10.1172/JCI40310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludin A, Itkin T, Gur-Cohen S, et al. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13:1072–1082. doi: 10.1038/ni.2408. [DOI] [PubMed] [Google Scholar]
- 5.Wagers AJ, Weissman IL. Differential expression of alpha2 integrin separates long-term and short-term reconstituting Lin-/loThy1.1(lo)c-kit+ Sca-1+ hematopoietic stem cells. Stem Cells. 2006;24:1087–1094. doi: 10.1634/stemcells.2005-0396. [DOI] [PubMed] [Google Scholar]
- 6.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abkowitz JL, Robinson AE, Kale S, et al. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102:1249–1253. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 8.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz N, Dreger P, Linch DC, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. The Lancet. 1996;347:353–357. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- 11.Adler BK, Salzman DE, Carabasi MH, et al. Fatal sickle cell crisis after granulocyte colony-stimulating factor administration. Blood. 2001;97:3313–3314. doi: 10.1182/blood.v97.10.3313. [DOI] [PubMed] [Google Scholar]
- 12.Kollet O, Spiegel A, Peled A, et al. Rapid and efficient homing of human CD34(+)CD38(−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 13.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 15.Golan K, Vagima Y, Ludin A, et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119:2478–2488. doi: 10.1182/blood-2011-06-358614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoggatt J, Mohammad KS, Singh P, et al. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature. 2013;495:365–369. doi: 10.1038/nature11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juarez JG, Harun N, Thien M, et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119:707–716. doi: 10.1182/blood-2011-04-348904. [DOI] [PubMed] [Google Scholar]
- 18.Ratajczak MZ, Lee H, Wysoczynski M, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogle ME, Sefcik LS, Awojoodu AO, et al. Engineering in vivo gradients of sphingosine-1-phosphate receptor ligands for localized microvascular remodeling and inflammatory cell positioning. Acta Biomater. 2014;10:4704–4714. doi: 10.1016/j.actbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, et al. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 22.Allende ML, Sasaki T, Kawai H, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 23.Awojoodu AO, Ogle ME, Sefcik LS, et al. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A. 2013;110:13785–13790. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter DH, Rochwalsky U, Reinhold J, et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–282. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- 25.Baudhuin LM, Jiang Y, Zaslavsky A, et al. S1P3-mediated Akt activation and cross-talk with platelet-derived growth factor receptor (PDGFR) FASEB J. 2004;18:341–343. doi: 10.1096/fj.03-0302fje. [DOI] [PubMed] [Google Scholar]
- 26.Krieger JR, Ogle ME, McFaline-Figueroa J, et al. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1alpha-releasing hydrogels enhances microvascular network remodeling. Biomaterials. 2016;77:280–290. doi: 10.1016/j.biomaterials.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Bernal D, Wright N, Sotillo-Mallo E, et al. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin alpha4beta1. Mol Biol Cell. 2005;16:3223–3235. doi: 10.1091/mbc.E04-12-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, Das A, Barker D, et al. Local delivery of FTY720 accelerates cranial allograft incorporation and bone formation. Cell Tissue Res. 2012;347:553–566. doi: 10.1007/s00441-011-1217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma MB, Limaye LS, Kale VP. Mimicking the functional hematopoietic stem cell niche in vitro: recapitulation of marrow physiology by hydrogel-based three-dimensional cultures of mesenchymal stromal cells. Haematologica. 2012;97:651–660. doi: 10.3324/haematol.2011.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright DE, Bowman EP, Wagers AJ, et al. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Van Brocklyn JR, Hobson JP, et al. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeldt HM, Hobson JP, Maceyka M, et al. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J. 2001;15:2649–2659. doi: 10.1096/fj.01-0523com. [DOI] [PubMed] [Google Scholar]
- 33.Zhu R, Snyder AH, Kharel Y, et al. Asymmetric synthesis of conformationally constrained fingolimod analogues–discovery of an orally active sphingosine 1-phosphate receptor type-1 agonist and receptor type-3 antagonist. J Med Chem. 2007;50:6428–6435. doi: 10.1021/jm7010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MJ, Van Brocklyn JR, Thangada S, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 35.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 36.Buitenhuis M, van der Linden E, Ulfman LH, et al. Protein kinase B (PKB/c-akt) regulates homing of hematopoietic progenitors through modulation of their adhesive and migratory properties. Blood. 2010;116:2373–2384. doi: 10.1182/blood-2009-10-250258. [DOI] [PubMed] [Google Scholar]
- 37.Hale JJ, Lynch CL, Neway W, et al. A rational utilization of high-throughput screening affords selective, orally bioavailable 1-benzyl-3-carboxyazetidine sphingosine-1-phosphate-1 receptor agonists. J Med Chem. 2004;47:6662–6665. doi: 10.1021/jm0492507. [DOI] [PubMed] [Google Scholar]
- 38.Sugrue T, Brown JA, Lowndes NF, et al. Multiple facets of the DNA damage response contribute to the radioresistance of mouse mesenchymal stromal cell lines. Stem Cells. 2013;31:137–145. doi: 10.1002/stem.1222. [DOI] [PubMed] [Google Scholar]
- 39.Lo Celso C, Fleming HE, Wu JW, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. The Journal of Clinical Investigation. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frasca D, Guidi F, Arbitrio M, et al. Hematopoietic reconstitution after lethal irradiation and bone marrow transplantation: effects of different hematopoietic cytokines on the recovery of thymus, spleen and blood cells. Bone Marrow Transplant. 2000;25:427–433. doi: 10.1038/sj.bmt.1702169. [DOI] [PubMed] [Google Scholar]
- 42.Ishii M, Kikuta J, Shimazu Y, et al. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207:2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura T, Boehmler AM, Seitz G, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103:4478–4486. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- 44.Hale JJ, Yan L, Neway WE, et al. Synthesis, stereochemical determination and biochemical characterization of the enantiomeric phosphate esters of the novel immunosuppressive agent FTY720. Bioorg Med Chem. 2004;12:4803–4807. doi: 10.1016/j.bmc.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu T, De Wispelaere A, Winkler M, et al. Sphingosine-1-phosphate receptor 3 promotes neointimal hyperplasia in mouse iliac-femoral arteries. Arterioscler Thromb Vasc Biol. 2012;32:955–961. doi: 10.1161/ATVBAHA.111.241034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitson SM, Moretti PA, Zebol JR, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wysoczynski M, Reca R, Ratajczak J, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 48.Singleton PA, Dudek SM, Ma SF, et al. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen DH, Taub D. CXCR4 function requires membrane cholesterol: implications for HIV infection. J Immunol. 2002;168:4121–4126. doi: 10.4049/jimmunol.168.8.4121. [DOI] [PubMed] [Google Scholar]
- 50.Kim CH, Wu W, Wysoczynski M, et al. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106–116. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim C, Schneider G, Abdel-Latif A, et al. Ceramide-1-phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells–implications for tissue regeneration. Stem Cells. 2013;31:500–510. doi: 10.1002/stem.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peled A, Grabovsky V, Habler L, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casanova-Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen H, Cheng T, Olszak I, et al. CXCR-4 desensitization is associated with tissue localization of hemopoietic progenitor cells. J Immunol. 2001;166:5027–5033. doi: 10.4049/jimmunol.166.8.5027. [DOI] [PubMed] [Google Scholar]
- 55.Kim MH, Granick JL, Kwok C, et al. Neutrophil survival and c-kit(+)-progenitor proliferation in Staphylococcus aureus-infected skin wounds promote resolution. Blood. 2011;117:3343–3352. doi: 10.1182/blood-2010-07-296970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Bryder D, Adolfsson J, et al. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 58.Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 59.Nervi B, Ramirez P, Rettig MP, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng Z, Shi YX, Samudio IJ, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) During homeostasis, high local SDF-1 concentrations signals through CXCR4 to phosphorylate Akt and promote HSPC retention within the niche through increased adhesion. Low interstitial S1P concentration supports activation of Akt through S1PR3. (B) Antagonism of CXCR4 by AMD3100 reduces SDF-1-mediated retention of HSPCs within BM. AMD3100 also increases plasma S1P concentration enabling HSPCs to migrate towards S1P-rich plasma using S1PR1. Lower Akt activation supports egress rather than retention. (C) Antagonism of S1PR3 reduces local SDF-1 concentrations, decreasing signaling through CXCR4, and reduces total Akt phosphorylation, allowing HSPCs to follow S1P signals to the plasma through S1PR1. (D) In the presence of VPC01091 and AMD3100, both S1PR3- and CXCR4-mediated retention within the BM niche are blocked and S1PR1-mediated signaling promotes maximal egress.
Cells sorted as S1PR3 high and low populations were digested and analyzed by western blot for expression of S1PR3 protein. High and low populations produced the expected band intensity with an independent S1PR3 antibody. Whole bone marrow (WBM) was used as control.
(A, B) Mice treated with selective agonists/antagonists of S1PR1 and S1PR3 signaling demonstrate differential mobilization of LSK cells into peripheral blood after 1.5h (n>3 mice per group). (C) S1PR3 antagonism by VPC01091, but not S1PR1 agonism by SEW2871, alters the trafficking of white blood cells, including neutrophils and lymphocytes (n>4 mice per group). Data expressed as mean ± SEM. *p<0.05.
(A) BM expression of Sphk1, (B) SDF-1 intracellular BM protein, and (C) Sdf1 mRNA in the BM of mice 1.5h after VPC01091 treatment (n=15–16 mice). (D) Secretion of SDF-1 by OP9 MSCs 1.5h after FTY720 treatment as detected by ELISA (n=2–3 independent experiments). (E) SDF-1 staining (red) of OP9 MSC in 3D culture 1.5h after FTY720 treatment (1 μM) shows a decrease in cellular SDF-1 and increase in diffuse SDF-1 signal after FTY720 treatment indicating secretion of the protein. (Nuclei, blue; scale bar 50um)


