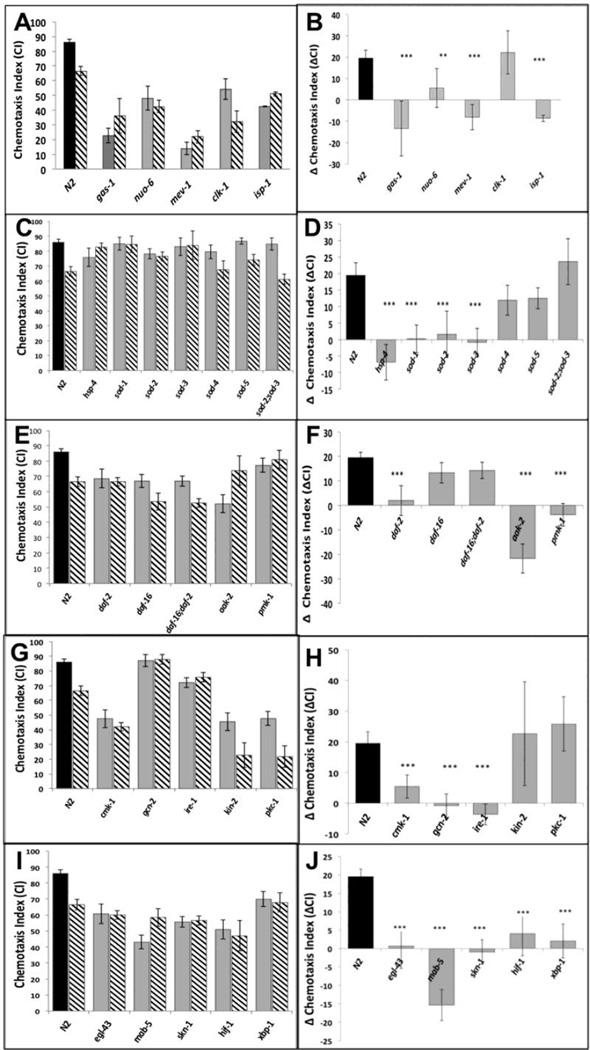
Figure 1.
A,C,E,G,I. Chemotaxis indices (CIs) in adults after exposure to isoflurane as L1 larvae. Unexposed animals (solid fill), exposed animals (angled hatching). For all graphs, error bars denote SEM values, N>300 animals for each value. B,D,F,H,J Differences in CIs (ΔCI) between exposed and unexposed animals. Difference between ΔCI of N2 (19.5 +/− 3.7) and each mutant was compared to determine if the mutant affected AIN. ** = ΔCI different from N2, p<0.01, ***= ΔCI different from N2, p<0.005. A,B. Mitochondrial mutants. Chemotaxis in unexposed mitochondrial mutants (gas-1, nuo-6, mev-1, isp-1) was not worsened by isoflurane exposure. The exception was clk-1 which had a ΔCI similar to that of N2. C,D. ROS scavengers/ ER Stress. The effects of defects in ROS scavenging on AIN in C. elegans. CIs of five superoxide disumutatse mutants and hsp-4 (loss of the ER-specific heat shock protein HSP-4). E,F. DAF-2 dependent pathway. The effects of the daf-2 stress pathway on AIN. Loss of DAF-2 removed the AIN effect. The daf-16 mutation removed the effect of daf-2 on AIN. G,H. Kinases. Effects of 5 kinases on neurotoxicity. Loss of cmk-1 and gcn-2, both involved in innate immunity and ER-related stress, eliminated AIN. Loss of ire-1 also eliminated AIN and is discussed later. I,J. Transcription factors. The transcription factors skn-1, hif-1 and xpb-1 all eliminated AIN.