Abstract
Purpose
The complexity of upper extremity (UE) behavior requires recovery of near normal neuromuscular function to minimize residual disability following a stroke. This requirement places a premium on spontaneous recovery and neuroplastic adaptation to rehabilitation by the lesioned hemisphere. Motor skill learning is frequently cited as a requirement for neuroplasticity. Studies examining the links between training, motor learning, neuroplasticity, and improvements in hand motor function are indicated.
Methods
This case study describes a patient with slow recovering hand and finger movement (Total Upper Extremity Fugl–Meyer examination score = 25/66, Wrist and Hand items = 2/24 on poststroke day 37) following a stroke. The patient received an intensive eight-session intervention utilizing simulated activities that focused on the recovery of finger extension, finger individuation, and pinch-grasp force modulation.
Results
Over the eight sessions, the patient demonstrated improvements on untrained transfer tasks, which suggest that motor learning had occurred, as well a dramatic increase in hand function and corresponding expansion of the cortical motor map area representing several key muscles of the paretic hand. Recovery of hand function and motor map expansion continued after discharge through the three-month retention testing.
Conclusion
This case study describes a neuroplasticity based intervention for UE hemiparesis and a model for examining the relationship between training, motor skill acquisition, neuroplasticity, and motor function changes.
Keywords: Stroke, upper extremity, virtual reality, robotics, rehabilitation, hand
Introduction
Despite over a decade of investigation of upper limb motor therapies, 78% of people poststroke continue to have upper extremity (UE) deficits that decrease their independence.[1] Some authors suggest this is due to the fact that the complexity of UE behavior requires recovery of neuromuscular function that approaches normal. Arguably, this high level of function decreases the value of compensatory strategies and abnormal contralesional control of the hemiparetic UE,[2] placing a premium on spontaneous recovery and neuroplastic adaptation to rehabilitation by the lesioned hemisphere. In turn, this establishes an imperative to design rehabilitation activities that are congruent with established requirements for neuroplasticity and studies that confirm the behavioral changes elicited by rehabilitation interventions occurring as a result of adaptive patterns of neuroplasticity.[3]
A key factor to consider during the planning of treatment focused on neuroplasticity following a stroke is timing. A large majority of the rehabilitation literature describing interventions targeting hand function in persons with stroke are conducted in persons during the chronic stage of recovery. Animal studies have shown that earlier rehabilitation, leads to greater preservation of the cortical areas controlling the hand in both the lesioned and non-lesioned hemispheres.[4,5] Similar studies in humans suggest that rehabilitation interventions presented in the first few weeks following a stroke may be more effective.[3] This case study will describe changes in lesioned motor cortex function occurring during the course of a neuroplasticity-based intervention, performed by a person in the subacute stage of stroke recovery.
Another key factor in the design of rehabilitation programs during this period is the amount of practice needed. Three hundred repetitions of UE activity has been identified as necessary to elicit neuro-plastic adaptations in persons with stroke.[6] Observational studies of typical rehabilitation sessions describe substantially less activity.[7] Constraint induced movement protocols elicit more than three hundred repetitions per hour.[8] Studies of CIMT, delivered to persons who are less than one month following a stroke in inpatient rehabilitation facilities, have established that this higher repetition arm and hand motor training is safe, feasible, and effective.[9] The simulations making up the intervention described in this paper are designed to deliver a large number of repetitions of activity efficiently.
Another requirement for behavior-dependent neuroplasticity is the continuous development of motor skill. In studies of animal and human response to behavioral interventions, changes in neural function are tied to changes in motor skill.[10] These changes level off when subjects continue to practice skills after they have been mastered.[11] This case presents a set of simulated rehabilitation activities controlled by algorithms that make the activity more challenging as soon as the current difficulty level is mastered. Our lab has utilized this approach successfully in the rehabilitation of persons with chronic [12] and it has been employed successfully in another lab’s study of persons with subacute stroke.[13] This case will utilize two approaches to the measurement of motor skill learning. The first is the measurement of performance during training tasks. The second is measurement of improvement in the ability of the fingers to perform untrained tasks.
This case study presents the responses of a subject with subacute stroke to an eight-session program of simulated activities in virtual reality (VR) that was designed to provide a strong stimulus for neuroplastic adaptation. The activities target recovery of the hand, arm, and fingers and utilize high repetitions of activities scaled to the patient’s movement abilities that are constantly progressed in difficulty. Training and transfer task kinematics are measured to document and confirm motor skill development. Transcranial magnetic stimulation (TMS) is utilized to describe plasticity of the brain structures controlling the fingers. Finally, clinical measures are presented to confirm the translation of these changes in motor skill and brain function into real-world movement ability.
Methods
Participant and protocol
AD was a 62 year-old right-handed female with a history of left pontine stroke with right hemiplegia, non-fluent aphasia and dysphagia, 37 days prior to her enrollment in the study. Her medical history was positive for uncontrolled hypertension and hyperthyroidism. AD was transferred to an Acute Rehabilitation Unit eight days after her stroke and received a standard inpatient rehabilitation program of one hour each of Occupational, Physical, and Speech Therapy, five days per week which started 9 days after her CVA. Prior to her enrollment in this study AD participated in 31 (30 min) sessions of Occupational Therapy. She began training in this study 37 days poststroke and also continued with her ongoing standard rehabilitation. AD returned to the community following her posttest. She completed 9 sessions of outpatient Occupational Therapy that focused on her UE prior to her retention testing session which occurred three months later.
AD was part of a larger sample of individuals recruited for a pilot study conducted at the Acute Rehabilitation Unit of St. Joseph’s Hospital in Wayne, NJ. The study protocol was approved by the Institutional Review Board of St. Joseph’s Hospital. AD performed a one-hour VR-based intervention eight times in a two-week period. All VR simulations were designed to facilitate highly repetitive practice combined with accuracy demands that required conscious attention to task. Training time was split evenly between simulations that addressed arm and shoulder movement and simulations that addressed hand and finger movement. The balance of this case report will address the VR-based interventions and outcomes related to the hand and fingers.
Hardware and software
The NJIT-Track Glove system consists of a CyberGlove™ (CyberGlove Systems San Jose, CA), which is an instrumented glove for finger angle tracking, and a TrackStar™ three-dimensional magnetic tracking system (Ascension Technology, Shelburne, VT) used to track hand position and orientation. The CyberGlove was used as an interface between AD and the virtual environments for three of the simulations used in this case study, Piano Trainer, Mirror Pong, and Space Pong. For the Piano Trainer and Mirror Pong simulations, the CyberGrasp device, a force reflecting exoskeleton that fits over a CyberGlove data glove was used to facilitate finger extension.[14,15] Pinch force training and testing was performed using an ATI Nano17™ force sensor (ATI Industrial Automation, Apex, NC). All the VR simulations were developed using Matlab (Mathworks, Nattick, MA), C++, VirtoolsTM (Dassault Systemes, Vélizy-Villacoublay Cedex, France), or an Open GL library. AD performed four simulations that addressed hand function, Piano Trainer, Space Pong, Mirror Pong, and Monkey Business.
Mirror Pong is utilized to prime the motor cortex to maximize the effectiveness of the three simulations that follow it. The participant utilizes a cyberglove worn on the unimpaired hand to control the virtual image of their impaired hand and a Cybergrasp worn on the paretic hand which passively extends the fingers. The participant views a virtual image of their paretic hand with their own hands covered by the viewing screen. The amount of movement of the paretic hand is yoked to the amount of movement of the unimpaired hand. In theory, priming of the motor cortex is accomplished via two mechanisms. Viewing a moving virtual image of the paretic hand controlled by the non-paretic hand has been linked to increased activity in the lesioned primary mortex cortex as measured by functional magnetic resonance imaging.[16] In addition, an extended period of repetitive passive movement of a paretic hand has been linked to upregulation of the lesioned motor cortex and improvements in motor performance, immediately following this period, in persons with stroke.[17] The simulation utilized in our intervention adds a game in which the virtual paretic hand is used to strike a moving target with each movement in order to increase conscious attention to the task.
Piano Trainer is designed to improve finger individuation. The simulation and its algorithm for shaping finger individuation are described in detail elsewhere.[15] Our approach to initiating training with facilitation from the Cybergrasp and weaning this facilitation over the course of the intervention is described in detail in our case report on a patient with chronic stroke.[18] Space Pong targets the control of mass finger flexion and extension. The subjects control a pong paddle using flexion and extension of the fingers measured with a CyberGlove, playing a game against the computer. Gain can be magnified during the performance of this simulation to allow a participant with as little as 3° of active finger movement to perform a meaningful activity. Gain magnification is modified on a daily basis to increase the challenge of this simulation as active finger movement abilities increase. Monkey Business targets control of a fore-finger and thumb, pinching movement in a fashion similar to Space Pong. The participant controls a monkey avatar, as it jumps to grab tree branches. Larger pinch forces result in higher jumping height by the monkey. Threshold of the load cell utilized to measure pinch force is small enough to allow participants with trace finger flexion and thumb adduction strength to participate in this activity. Pinch force is modified on a daily basis to increase the challenge of this simulation as finger strength increases.
TMS was used to assay changes in cortical neurophysiology by mapping the topographic representation of the finger-hand muscles (first dorsal interossei [FDI], adductor policis brevis [APB], extensor digitorum communicus [EDC]) before and after training and a third session three months after training. TMS mapping was performed using a Magstim Rapid2TM 70 mm double coil. Surface EMG was recorded using a 2 kHz Delsys TrignoTM system.
Motor mapping
TMS mapping of the motor cortex was performed by delivering TMS pulses over an expanse of the sensorimotor cortex and measuring the motor evoked potentials (MEPs) in the contralateral FDI, APB, and EDC muscles. Maps for the lesioned and non-lesioned hemispheres were collected. For the mapping procedure, AD was seated with her UE comfortably secured to limit motion. To ensure spatial TMS precision, AD’s high-resolution anatomical MRI was used to render a 3D cortical surface that was co-registered with her head to allow for frameless neuronavigation (Advanced Neuro Technology). For all stimuli, the TMS coil was held tangential to the scalp with the handle posterior 45° off the sagittal plane. We first identified the hotspot for the contralateral FDI, defined as the loci which produced the maximal MEP in the FDI muscle. Following this, the resting motor threshold (RMT) was calculated as the minimum intensity required to elicit MEPs >50 µV in the FDI muscle on 50% of six consecutive trials.[19] Stimulation intensity was set to 110% of the determined RMT [20] for mapping. A 10 × 10cm area surrounding the motor hotspot was marked using the neuronavigation software to provide consistent map boundaries. Real time visual feedback of the MEP time traces and neuronavigated coil position provided to the experimenter during testing maximized the map information obtained by allowing for increased density of points in excitable and border regions, with less attention given to far-away non-responsive areas.[21] For each stimulation point we computed the following measures: (i) MEP as the peak-to-peak amplitude of the EMG signal 20–50 ms after the TMS pulse, and (ii) background EMG, calculated as the EMG signal in the 50 ms interval before the TMS pulse (second order Butterworth filter, 5–250 Hz band-pass, full-wave rectified, 20 Hz low-pass envelope). A threshold of 50µV was used to identify MEPs from background EMG for the FDI and APB 36. MEPs were weaker for the EDC so a threshold of 25µV was used to generate this map. To allow comparisons across maps and sessions, MEP amplitudes and stimulation points were interpolated to a 10 × 10cm mesh of 5mm resolution centered on the M1 hotspot, using cubic surface interpolation.[22] Extent of the representation producing corticospinal output (MEPs), or map area, is calculated as the product of the number of interpolated scalp sites eliciting MEPs and the map resolution (0.5 mm).[22,23] Map area for each muscle is reported as the primary outcome measure for this study.
Clinical measurements
The Upper Extremity Fugl–Meyer Exam (UEFMA) is a 33 item battery, which evaluates reflexes, movement patterns, grasp, and coordination, higher scores indicate better performance.[24] The Wolf Motor Function Test (WMFT) is a 15-item battery that measures at the activity level.[25] A score of “unable” was recorded when the subject could not perform a task within 120 seconds. The ability to perform a test item within 120 seconds at a test subsequent to the pretest that a participant was previously unable to perform has been cited as a clinically meaningful change in persons with CVA.[26] The Box and Blocks Test (BBT) is a simple measure of unilateral gross manual dexterity. It measures the number of wooden blocks moved in one minute from one side of a box to the other.[27]
Kinematic assessment
All measures were collected with the patient seated with their trunks supported and their hands resting on a table. Performance for tracing tasks was measured following a single familiarization trial, which was not scored.
Finger range of motion was measured as the difference between all the joint angles with the fingers in a flexed position (fist) and the joint angles of all of the fingers extended (open) as much as possible. Finger angles were collected using a Cyberglove™ (Cyberglove Systems, San Jose CA). Larger numbers indicated increased active finger extension range of motion.
Finger Trace is the ability to control active finger extension and flexion between 0% and 80% of maximum finger extension, measured by having the subject flex and extend their fingers to control a cursor tracking a sine wave (duration of 1 cycle ≈6 s, period = 0.15 Hz). Smaller root mean square error (RMSE) values indicate improved performance.
Pinch force was measured as the maximum force a subject can exert on a force sensor held between their paretic thumb and index finger, given two trials. Larger numbers indicate stronger pinch force. This task was designed as a transfer test of motor learning accomplished during the Space Pong simualtion.
Pinch Trace measures the ability to control pinch force between 0% and 80% of maximum pinch force, measured by having the subject vary pinch grip force to control a cursor tracking a sine wave (duration of 1 cycle ≈6 s, period = 0.15 Hz). Smaller RMSE values indicate better performance. This task was designed as a transfer test of motor learning accomplished during the Monkey Business simualtion.
Results
Training kinematics
AD completed eight sessions of VR training with no adverse events, complaints of increased UE pain, or fatigue that limited her ability to complete the rest of her in-patient acute rehabilitation program. During the first session AD was extremely excited that she could “do something with her hand”. Her first session using the VR system was the first time since her stroke that she actively controlled her fingers during an activity. She did not miss a session during the trial and expressed a desire to use the equipment after the study period was completed. AD performed more than 300 repetitions in each of the eight sessions meeting the minimum repetition requirements for neuroplasticity. Two of the finger training simulations are relatively discrete activities that lend themselves to performance analysis during the training process.
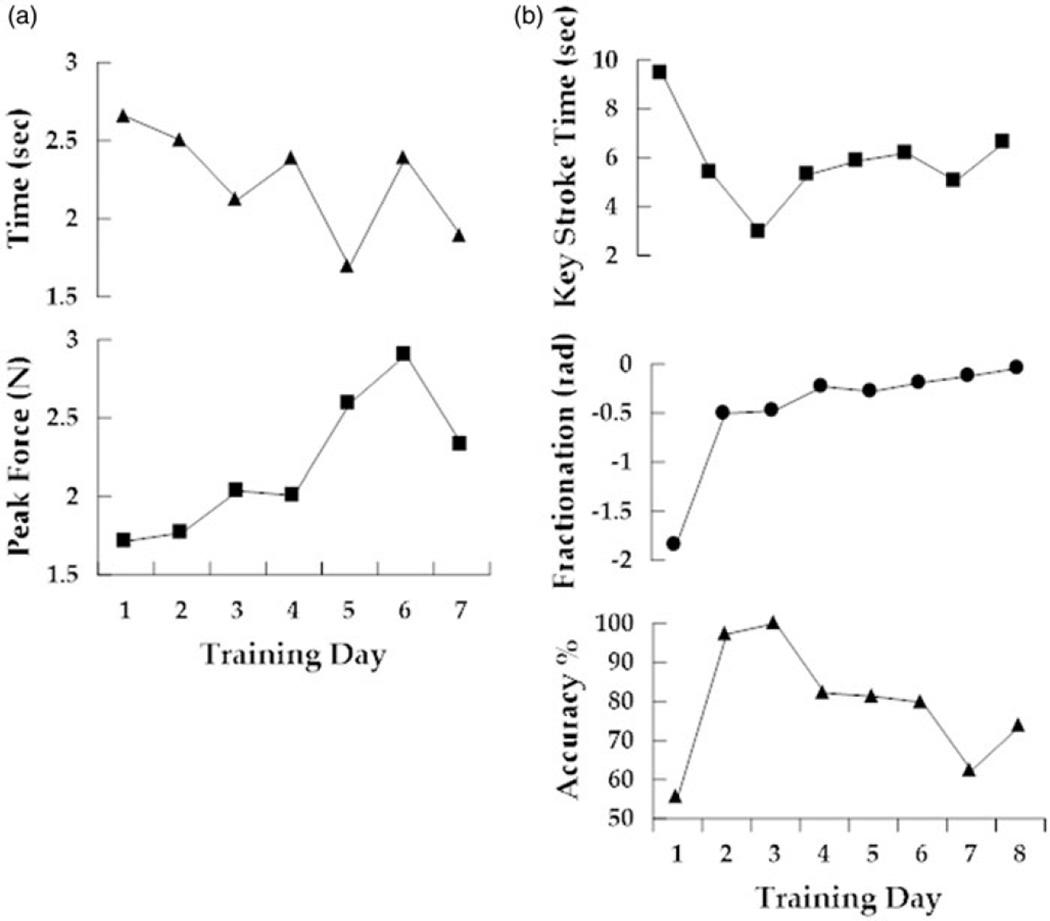
AD performed the Piano Trainer simulation on all eight training days (See Figure 1(a)). She required assistance from the CyberGrasp to maintain non-cued finger opening on training day one and three. This assistance was provided for 100% of the training period. Assistance from the CyberGrasp was provided for 50% of each session on training days four through six. No assistance was provided on days seven or eight. Time per key press was measured on each day. Time decreased steadily over the first three days with robotic assistance. Dramatic improvements in key press accuracy as well as fractionation (the targeted skill) occurred over these first three days as well. On days four through six the subject’s key press time became slower, particularly on day six as she struggled with individuated flexion of her ring finger. It is important to note that accuracy and fractionation continued to improve incrementally despite the substantial increase in difficulty. Key press time and accuracy changed little over the final two, no assistance training days. It is important to note that fractionation, the skill shaped by the algorithm, continued to demonstrate small incremental improvements each day despite the steady decrease in assistance.
Figure 1. Training performance.
(a) Average time to attain a target (top) versus peak force (bottom) recorded on seven consecutive training days during performance of the Monkey Business. (b) Average time to perform a keystroke (squares, top), the difference between flexion angles of cued and non-cued fingers (middle) and the number of correct keys pressed divided by the total number of keys pressed (traingles, bottom) recorded on eight consecutive training days during performance of the Piano Trainer simulation.
AD performed the Monkey Business simulation, on seven of the eight training days (See Figure 1(b)). Average time to attain a target, a measure of the accurate modulation of pinch force, was recorded. Each day the force required to move the monkey was increased, based on AD’s maximum isometric pinch force on that day. The time to attain targets improved on five of the seven days. Pinching force increased almost 50% over the course of the intervention. AD’s best performance occurred on day five for this simulation, demonstrating the best accuracy score and the second highest force score. Performance on both measures remained substantially better than baseline over the final two days.
Transfer test kinematics
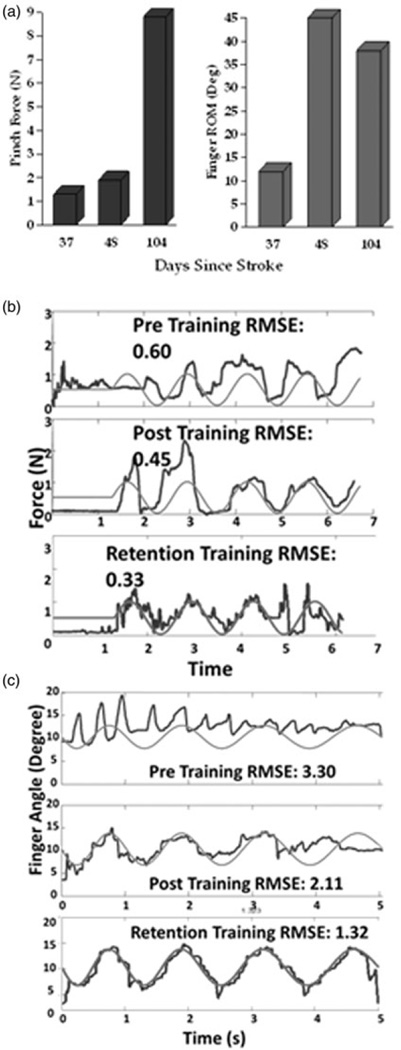
Improvements in AD’s ability to trace a sine wave by modulating pinch force improved 25% from pretest to posttest and another 30% from posttest to retention. Her ability to control the opening and closing of her hand to trace a sine wave followed a similar pattern with 25% improvements between pre and posttest and also between posttest and retention (see Figures 2(a) and 2(b)). Both of these sets of improvements occurred in spite of the fact that she did not perform sine wave tracing tasks during the experimental training activities or the retention period.
Figure 2. Untrained transfer task kinematics.
(a) Changes in max pinch force and active finger extension range of motion. (b) Attempts to trace red sine wave controlling cursor by pinching force sensor. Measured preintervention (top), postintervention (middle) and at 3 month retention (bottom). (c) Attempts to trace red sine wave controlling cursor by extending (up) and flexing (down) fingers as measured with a strain-gauge glove. Measured preintervention (top), postintervention (middle) and at 3 month retention (bottom).
Motor mapping
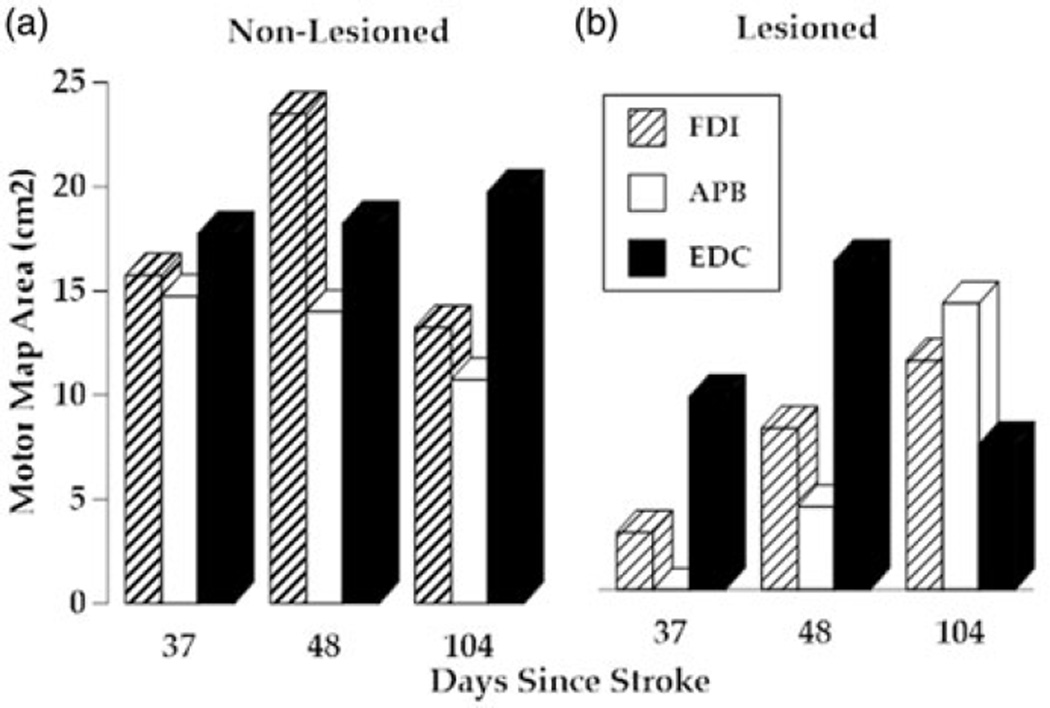
The area of the motor cortex eliciting MEPs at the FDI muscle expanded more than 100% from pre- to posttest and continued to expand during the retention period. Motor map changes for the non-lesioned hemisphere controlling the FDI increased from pre to posttest and then decreased substantially at retention (See Figure 3). Although no APB muscle MEPs were detected in lesioned hemisphere during pretesting, a 4cm2 APB map area was evident at posttesting, and tripled in size at retention testing. This large expansion of the APB map coincided with a large increase in pinch force measured at retention (see Figure 2). Motor map of the non-lesioned hemisphere for the APB decreased 1 cm2 from pre to posttest and then decreased by an additional 3 cm2 during the retention period. The lesioned hemisphere motor map of the EDC increased from 9cm2 to 16cm2 between pre and posttesting and then decreased to 7 cm2 at retention testing. This was accompanied by a gradual increase in map area for the EDC on the non-lesioned hemisphere. Interestingly, behavioral improvements for this muscle group, as measured by finger extension active range of motion and error related to sine wave tracing accomplished with finger extension (see Figure 4) made their largest improvements during the intervention and leveled off or regressed slightly during the retention period.
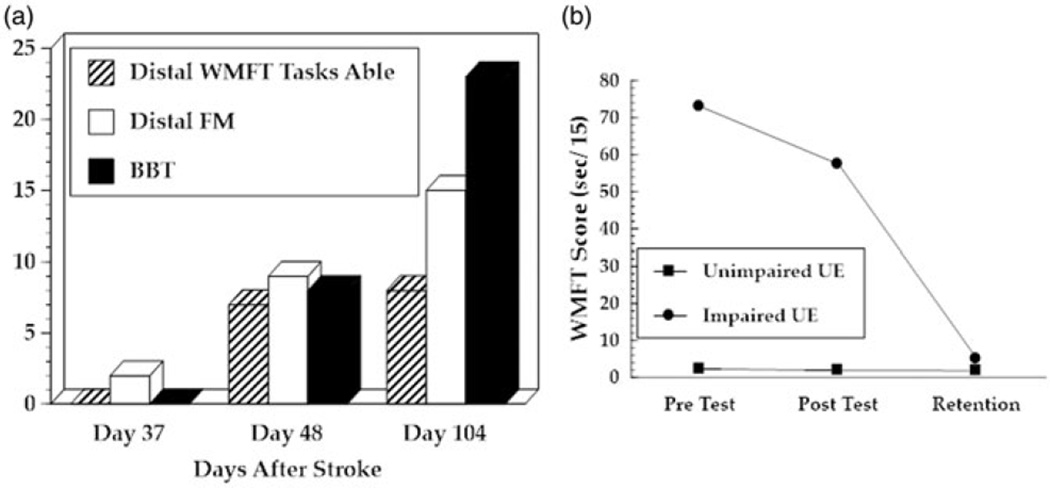
Figure 3. Clinical assessment.
(a) AD’s performance on the WMFT, UEFMA and BBT. Performances were measured 1 day before intervention, 1 day after intervention and 3 months after intervention. (b) Average time to complete each of the 15 items of the Wolf motor function test. Unimpaired UE performance (blue boxes) and impaired UE performance (red circles) measured on same days as (a).
Figure 4. Motor maps.
(a) Non-lesioned hemisphere motor map area for the first dorsal interosseous muscle (blue columns), the adductor pollicis brevis (red columns) and extensor digitorum communis (green columns). Data collection occurred on the same day as the clinical and kinematic measures from Figures 2 and 3. (b) Lesioned hemisphere motor map area for the same muscles collected on same days.
Clinical measurements
AD demonstrated an increase in total UEFMA score of 12 points from pretest to posttest which exceeds the MCID for persons with subacute stroke.[28] AD’s score increased an additional six points at retention testing. A substantial portion of the 18-point increase occurred in the hand subsection of the UEFMA with an increase of five points (of a possible 14) recorded during the intervention and an additional three point improvement (total increase = 8) recorded at retention. It is important to note that the five point pre to posttest increase occurring over the 12 day intervention period was larger than the improvement demonstrated during the first 49 days of recovery as well as the improvement demonstrated over the 56 day retention period (See Figure 3(a)).
At pretesting, AD was unable to perform any of the seven activities that required object manipulation in the WMFT. At posttesting she was capable of performing all but one of these items. At retention testing, AD was able to perform all of the WMFT tests (See Figure (a)). This improvement would qualify as meaningful based on the criteria described by Wolf et al. [25]. This pattern of functional recovery that persisted after completion of the intervention is similar to the pattern observed in our studies of simulated motor interventions in persons with more chronic strokes.
Motor performance tests are influenced by many non-motor factors including receptive communication, visual perception, and cognitive constructs including (but not limited to) arousal, attention, and working memory. A rough, clinical method to control for these non-motor factors is to observe changes in the motor performance of the less impaired UE. A sample of 10 healthy females between the ages of sixty and sixty-nine demonstrated an average composite WMFT of 1.28 ± (0).03 s with their dominant right hand and 1.33 ± (0).03 s with their non-dominant left hands. AD scored 2.3 s with her unimpaired, previously non-dominant left hand at pretest, suggesting that non-motor factors had a measurable but small impact on her WMFT performance with either hand.[29] Between pre and posttest, AD demonstrated a 0.6 s improvement with her less impaired hand and a 15.5 second improvement with her impaired UE. This would suggest that a majority of the performance improvement demonstrated with AD’s impaired hand could be attributed to improvements in motor control (See Figure 2(b)). During BBT performance, AD was unable to complete a single block at pretesting and increased to eight blocks at posttesting and 23 blocks at retention testing. This 15-block increase exceeds the MCID of 12 blocks (See Figure 2(a)).
Discussion
The large volume of training accomplished by AD during the intervention period and clear evidence that motor learning had occurred, confirms that important requirements for neuroplasticity were achieved. Expansion of lesioned hemisphere motor maps controlling the thumb and fingers combined with substantial improvements in hand function measured at the impairment and activity level are consistent with adaptive neuroplasticity as well. A great deal of these positive changes could be ascribed to spontaneous recovery. This said, the gradual training performance changes, and rapid acceleration of finger movement recovery occurring in the same week that the trial was initiated, support the possibility that training impacted the recovery of finger function. Several factors related to the training program and the relationship between changes in motor maps and clinical results suggest possible avenues for future study.
Two of the factors limiting the amount of intervention targeting the hand immediately following a stroke include: (a) challenges associated with translating minimal volitional movement into meaningful activities, and (b) precisely quantifying improvements in motor performance when volitional movement is minimal. The highly sensitive interfaces used in the experimental intervention overcome these challenges by allowing patients to turn trace finger movements into a variety of meaningful simulated tasks and recording highly precise kinematic and kinetic data that can detect incremental performance improvements. We argue that these advances may be integral not only for the optimization of interventions, but will also be useful for tracking the impact of interventions for clinical reimbursement.
The simulations performed by AD in this intervention were designed to maximize motor skill learning. AD demonstrated motor skill development when performing the piano training simulation as measured by fractionation changes (see Figure 1(b)) in a pattern comparable to our subjects with chronic stroke [15,18] despite her lower level of active finger movement at the beginning of the study. She demonstrated comparable improvements in accuracy when performing the Monkey Business simulation as well. Transfer of this learning to a non-trained task, the sine wave pinch-trace task support that motor skill learning occurred. A similar transfer of learning from the Space Pong training task to the un-trained finger-trace task adds to our case that our approach to training elicits changes in motor skill.
The development of motor skill demonstrated by AD over the course of the study period was accompanied by an increase in the ipsilesional cortical territory controlling the FDI and APB muscles as measured by TMS mapping. Higher levels of ipsilesional motor cortex activation (as measured by TMS) during control of the paretic UE are associated with better motor performance in persons with stroke.[30] Conversely, increased excitability of the contralesional hemisphere has been associated with poorer recovery of the impaired hand in the subacute stage.[31] Notably, no increase in the contralesional territory was found in either muscle at the one month retention compared to baseline. On the lesioned side, it is interesting to note that the ipsilesional motor cortex adaptations related to EDC (finger extensor) control did not demonstrate the strong consistent pattern of increased ipsilesional hemisphere territory. Contralesional motor map area for EDC gradually increased across the three measurement periods as well. The corresponding impairment and skill improvements related to finger extension were less pronounced and finger extension AROM actually regressed during the retention period (See Figures 2a and 2c). Continued study of the relationships between lesioned and non-lesioned hemispheres and their contributions to the control of the UE following a stroke may add important insight to guide the development of treatment programs for patients during the first few weeks after stroke.
The motor map expansion for the APB that accompanied large improvements in pinch force, modulation of pinch force, and clinical test scores was an interesting finding. Thumb movement is not typically a high priority during UE rehabilitation of persons with a CVA and was not emphasized during her rehabilitation program prior to the experimental intervention. This said, AD was able to form three of the four grasps tested by the UEFMA (all of which involve the thumb) following eight sessions of the experimental intervention which included 10 min of training targeting the thumb per session. Thumb motor map changes have been associated with improvements in motor performance in persons performing CIMT during the subacute stage of recovery from stroke. In addition, activity level improvement in the WMFT during the retention period may have been catalyzed by the initial activation of thumb movement accomplished during the experimental intervention. This said, AD’s continued motor-function recovery following targeted hand and finger training was consistent with that demonstrated by subjects in our previous studies of persons with strokes.[12] Further investigation of rehabilitation targeted at motor control of the thumb following stroke could have substantial impact on the treatment of this population.
The most obvious limitation of this study is the inability to distinguish between improvements in motor function elicited by training and those that occurred as a result of spontaneous recovery. This said, AD started the intervention 37 days after her stroke when spontaneous functional recovery [32] and spontaneous motor map expansion have typically leveled-off. A large RCT will be required to determine additive effects of this or any intervention to the normal process of recovery.
This case presents positive gains at the clinical, kinematic, and neurophysiological levels in a subacute stroke patient exposed to an intensive augmentative therapy aimed at the restoration of finger-hand movement. The data provide preliminary support for the feasibility of adding this type of training to inpatient rehabilitation training and raise potentially interesting avenues of investigation related to the design of interventions for this population and assaying outcomes at multiple system levels.
IMPLICATIONS FOR REHABILITATION.
Intensive hand and finger rehabilitation activities can be added to an in-patient rehabilitation program for persons with subacute stroke.
Targeted training of the thumb may have an impact on activity level function in persons with upper extremity hemiparesis.
Untrained transfer tasks can be utilized to confirm that training tasks have elicited motor learning.
Changes in cortical motor maps can be used to document changes in brain function which can be used to evaluate changes in motor behavior persons with subacute stroke.
Acknowledgments
The authors thank the entire staff of the Acute Rehabilitation Unit of Saint Joseph’s Wayne Hospital for their gracious assistance in all aspects throughout this project.
Funding
National Institutes of Health, 10.13039/100000002 [K01HD059983,R01-HD58301,R01-NS085122-01]
Footnotes
Disclosure statement
The authors state that they have no conflicts of interest.
References
- 1.Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil. 1999;21:258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- 2.Lang CE, Wagner JM, Edwards DF, et al. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair. 2006;20:444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- 3.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the post-stroke brain. Curr Opin Neurol. 2013;26:609. doi: 10.1097/WCO.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbay S, Plautz EJ, Friel KM, et al. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp Brain Res. 2006;169:106–116. doi: 10.1007/s00221-005-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J. Neurosci. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair. 2010;24:620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther. 2007;31:3–10. doi: 10.1097/01.npt.0000260568.31746.34. [DOI] [PubMed] [Google Scholar]
- 8.Abdullahi A. Is time spent using constraint induced movement therapy an appropriate measure of dose? A critical literature review. Int J Ther Rehabil. 2014;21:140–146. [Google Scholar]
- 9.Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke. 2000;31:2984–2988. doi: 10.1161/01.str.31.12.2984. [DOI] [PubMed] [Google Scholar]
- 10.Remple MS, Bruneau RM, VandenBerg PM, et al. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res. 2001;123:133–141. doi: 10.1016/s0166-4328(01)00199-1. [DOI] [PubMed] [Google Scholar]
- 11.Boyd LA, Vidoni ED, Wessel BD. Motor learning after stroke: is skill acquisition a prerequisite for contralesional neuroplastic change? Neurosci Lett. 2010;482:21–25. doi: 10.1016/j.neulet.2010.06.082. [DOI] [PubMed] [Google Scholar]
- 12.Fluet GG, Merians AS, Qiu Q, et al. Comparing integrated training of the hand and arm with isolated training of the same effectors in persons with stroke using haptically rendered virtual environments, a randomized clinical trial. J Neuroeng Rehabil. 2014;11:1. doi: 10.1186/1743-0003-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silva Cameir ã o M, Berm ú dez i Badia S, Duarte E, et al. MJ. Virtual reality based rehabilitation speeds up functional recovery of the upper extremities after stroke: a randomized controlled pilot study in the acute phase of stroke using the Rehabilitation Gaming System. Restor Neurol Neurosci. 2011;29:287–298. doi: 10.3233/RNN-2011-0599. [DOI] [PubMed] [Google Scholar]
- 14.Boos A, Qiu Q, Fluet GG, et al. Haptically facilitated bimanual training combined with augmented visual feedback in moderate to severe hemiplegia. Paper presented at: Engineering in Medicine and Biology Society, EMBC, Annual International Conference of the IEEE2011; 2011; Boston, MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamovich SV, Fluet GG, Mathai A, et al. Design of a complex virtual reality simulation to train finger motion for persons with hemiparesis: a proof of concept study. J Neuroeng Rehabil. 2009;6:28. doi: 10.1186/1743-0003-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saleh S, Qinyin Q, Adamovich S, et al. fMRI study of the effects of visual feedback manipulation on sensorimotor circuits. Paper presented at: Bioengineering Conference, Proceedings of the 2010 IEEE 36th Annual Northeast; 2010 March 26–28; New York, NY. [Google Scholar]
- 17.Fischer HC, Stubblefield K, Kline T, et al. Hand rehabilitation following stroke: a pilot study of assisted finger extension training in a virtual environment. Top Stroke Rehabil. 2007;14:1–12. doi: 10.1310/tsr1401-1. [DOI] [PubMed] [Google Scholar]
- 18.Fluet GG, Merians AS, Qiu Q, et al. Robots integrated with virtual reality simulations for customized motor training in a person with upper extremity hemiparesis: a case study. J Neurol Phys Ther. 2012;36:79–86. doi: 10.1097/NPT.0b013e3182566f3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler AJ, Kahn S, Wolf SL, et al. Finger extensor variability in TMS parameters among chronic stroke patients. J Neuroeng Rehabil. 2005;2:1. doi: 10.1186/1743-0003-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngomo S, Leonard G, Moffet H, et al. Comparison of transcranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. J Neurosci Methods. 2012;205:65–71. doi: 10.1016/j.jneumeth.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Niskanen E, Julkunen P, Säisänen L, et al. Group-level variations in motor representation areas of thenar and anterior tibial muscles: Navigated Transcranial Magnetic Stimulation Study. Hum Brain Mapp. 2010;31:1272–1280. doi: 10.1002/hbm.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghetti D, Sartucci F, Petacchi E, et al. Transcranial magnetic stimulation mapping: a model based on spline interpolation. Brain Res Bull. 2008;77:143–148. doi: 10.1016/j.brainresbull.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Brott T, Adams H, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 24.Deakin A, Hill H, Pomeroy VM. Rough guide to the Fugl-Meyer assessment, Upper limb section. Physiotherapy. 2003;89:751–767. [Google Scholar]
- 25.Wolf SL, Thompson PA, Morris DM, et al. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 26.Wolf S, Winstein C, Miller J, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 27.Mathiowetz V, Volland G, Kashman N, et al. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 28.Narayan Arya K, Verma R, Garg R. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil. 2011;18:599–610. doi: 10.1310/tsr18s01-599. [DOI] [PubMed] [Google Scholar]
- 29.Wolf SL, McJunkin JP, Swanson ML, et al. Pilot normative database for the Wolf Motor Function Test. Arch Phys Med Rehabil. 2006;87:443–445. doi: 10.1016/j.apmr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Boake C, Noser EA, Ro T, et al. Constraint-Induced Movement Therapy During Early Stroke Rehabilitation. Neurorehabil Neural Repair. 2007;21:14–24. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- 31.Mohapatra S, Harrington R, Chan E, et al. Role of contralesional hemisphere in paretic arm reaching in patients with severe arm paresis due to stroke: a preliminary report. Neurosci. Lett. 2016;617:52–58. doi: 10.1016/j.neulet.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22:281–299. [PubMed] [Google Scholar]