Figure 2.
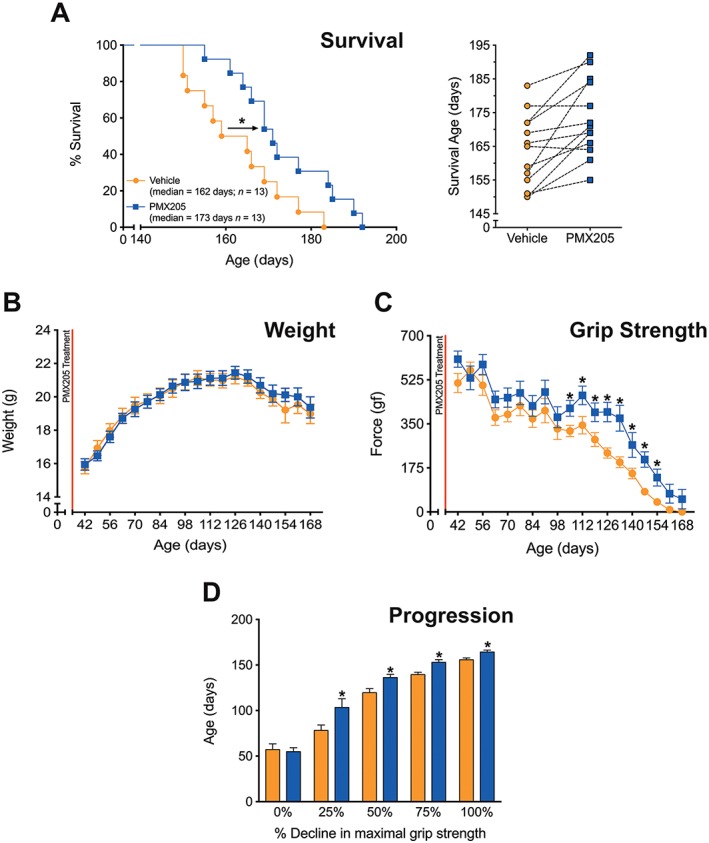
‘Pre‐onset’ PMX205 treatment at 9 mg·kg−1·day−1 in hSOD1G93A transgenic mice. hSOD1G93A mice were orally dosed with the selective C5a1 receptor antagonist PMX205 at 35 days of age (9 mg·kg−1·day−1 in drinking water through to end‐stage). (A, left panel) A Kaplan–Meier plot of ages (in days) in which hSOD1G93A mice treated with dH2O (vehicle) or PMX205 reached end‐stage of disease (complete hindlimb paralysis and an inability to right itself once placed on its back (n = 13). * P < 0.05, significantly different from vehicle; log‐rank test. (A, right panel) The end‐stage survival age for each litter‐matched pair of vehicle‐ and PMX205‐treated hSOD1G93A mice. (B) Body weight in vehicle‐ and PMX205‐treated hSOD1G93A mice (n = 13). P > 0.05, significantly different from vehicle; two‐way ANOVA. (C) Hindlimb grip strength in hSOD1G93A mice treated with either vehicle or PMX205 (n = 13) *P < 0.05, significantly different from vehicle; two‐way ANOVA with Fisher's LSD test at each age. (D) Disease progression (determined by age at which maximal grip strength decline at 25, 50, 75 and 100%) in hSOD1G93A mice treated with PMX205 when compared with vehicle‐treated hSOD1G93A mice at 25, 50, 75 and 100% decline in maximal grip strength (n = 13). * P < 0.05, significantly different from vehicle;two‐way ANOVA with Fisher's LSD test at each quartile. Data are expressed as mean ± SEM.
