Figure 9.
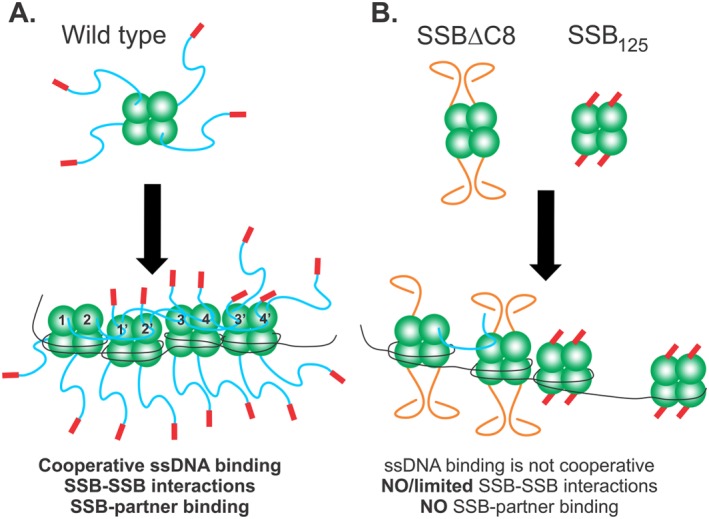
The IDL coordinates protein‐protein interactions to facilitate efficient SSB function. Schematics of wild type and mutant SSB proteins binding to ssDNA. The coloring of SSB monomers follows that of Figure 1, with the core domain in green, linker in blue and acidic tip in red. (A) Wild type protein establishes an IDL/OB‐fold network of interactions. Here tetramers have their functional C‐terminal domains exposed in solution. Upon binding to ssDNA in SSB35 mode for example, the IDLs of monomers 1 and 2, bind to the OB‐folds of monomers 1′ and 2′, respectively. Concurrently, the linkers of monomers 1′ and 2′ bind to the OB‐folds of monomers 3 and 4, and their IDLs bind to monomers 3′ and 4′, respectively. The C‐termini of subunits 3′ and 4′ are available to bind to an incoming tetramer. On the opposite side of each tetramer, C‐termini are available for binding to interactome partners. For simplicity, SSB‐SSB interactions are shown in the top subunits only. (B) SSBΔC8 and SSB125 mutant proteins have defective C‐termini and cannot establish an IDL/OB‐fold network of interactions. For SSBΔC8, the acidic tip is absent and as a result, linkers adopt a configuration that is incompatible with OB‐fold binding. Consequently, and even though this protein can still bind to ssDNA, co‐operativity is diminished, some IDL/OB‐fold interactions occur but binding to target proteins is eliminated. For SSB125 the linker is absent, but the acidic tip is retained. These tetramers cannot interact with other proteins but still retain the ability to bind ssDNA as the N‐terminal domains containing the OB‐folds are unaffected.
