Figure 4.
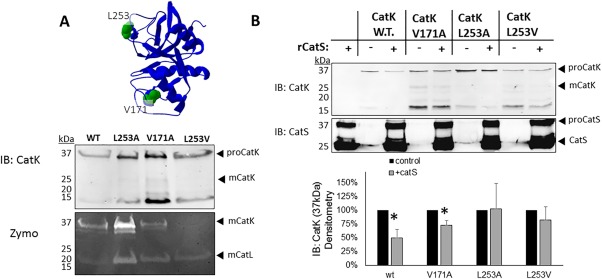
Expression of cleavage‐site cathepsin K mutants yield full length protein and are protected from degradation by cathepsin S. (A) HEK293T cells were stably transfected with a plasmid for each of the His‐tagged cathepsin K mutants. Cell pellets were collected and lysed and prepared for immunoblotting to determine if protein was produced, and cathepsin zymography to test for active cathepsin K with the mutations. Active cathepsin K in zymography yields a signal at 37 kDa under non‐reducing conditions, but that same sample run under reducing conditions would yield a 29 kDa protein detected in the Western blot. (B) One picomole of recombinant cathepsin S was co‐incubated with purified cathepsin K wildtype or cleavage site cathepsin K mutants for 2 hours at 37°C. Cathepsin S degraded 50% of wildtype (w.t.) cathepsin K (n = 3, P < 0.05). Mutations at V171 showed partial protection of 25% (n = 3, P < 0.05), but mutations at L253 protected cathepsin K with no statistically significant difference, in the presence or absence of cathepsin S (n = 3). Densitometry is quantified in the graph below.
