Figure 9.
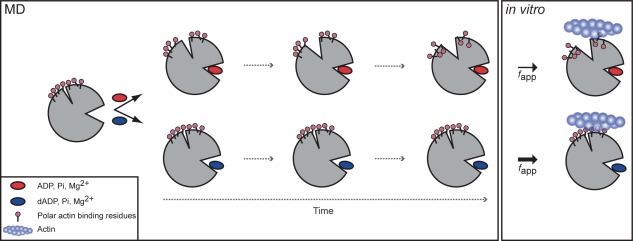
Schematic of dADP.Pi‐induced changes to myosin structure. Simplified overview of allosteric pathway of alterations over time to myosin structure with ADP.Pi (top) vs. dADP.Pi (bottom). ADP.Pi was stable in the binding pocket and maintained contacts with the switch regions, which stabilized a “closed” conformation of the binding pocket. This resulted in an opening of the actin binding cleft over time, which in turn reduced the exposure of polar residues on the actin‐binding surface. dADP.Pi, on the other hand, destabilized the binding pocket. Contacts were broken between the switch regions and the nucleotide, Pi and Mg2+, which stabilized an “open” conformation of the nucleotide‐binding pocket resulting in increased SASA of the nucleotide. This binding pocket conformation stabilized a closed cleft conformation, which in turn favored increased (relative to ADP.Pi simulations) exposure of charged residues on the actin binding surface of myosin bound to dADP.Pi. The model predicted that the increased charge on the actin binding surface would result in increased electrostatic interactions between myosin and actin, which was supported by the IVM data (Fig. 9) and previous in vitro studies,7 and suggests a mechanism by which dATP (and its hydrolysis products, dADP and Pi) increase actomyosin interaction and force production.
