Abstract
Hematopoietic stem cells (HSCs) have the capacity to self-renew and differentiate into hematopoietic cells and have been utilized to replace diseased bone marrow for patients with cancers and blood disorders. Although remarkable progress has been made in developing new tools to manipulate HSCs for clinic use, there is still no effective method to expand HSCs in vivo for quick repopulation of hematopoietic cells following sublethal irradiation. We have recently described a novel synthetic cytokine that is derived from the fusion of granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4; named as GIFT4), and we have now discovered that GIFT4 fusokine promotes long-term hematopoietic regeneration in a B cell-dependent manner. We found that GIFT4 treatment triggered a robust expansion of endogenous bone marrow HSCs and multipotent progenitors in vivo. Delivery of GIFT4 protein together with B cells rescued lethally irradiated mice. Moreover, adoptive transfer of autologous or allogeneic GIFT4-treated B cells (GIFT4-B cells) enhanced long-term hematopoietic recovery in radiated mice and prevented the mice from irradiation-induced death. Our data suggest that GIFT4 as well as GIFT4-B cells could serve as means to augment HSC engraftment in the setting of bone marrow transplantation for patients with hematological malignancy.
Keywords: hematopoietic stem cells, B cells, fusion cytokine
Deng et al. show that a GM-CSF and IL-4 fusion cytokine induce B cell-dependent expansion of endogenous hematopoietic stem cells and multipotent progenitors, which promotes long-term hematopoietic regeneration in a preclinical bone marrow failure animal model. The findings provide conceptual insight into a link between B cells and hematopoiesis.
Introduction
Hematopoietic stem cells (HSCs) have the capacity to self-renew and differentiate into all kinds of hematopoietic and immune cells,1 even non-blood cells.2 As a portion of lineage-negative (Lin−), Sca-1 (stem cells antigen-1)-positive and c-Kit (CD117)-positive cells (LSK cells) containing hematopoietic stem and progenitor cell pool,3, 4 HSCs primarily reside in a highly complex microenvironment of the bone marrow.5, 6 Due to their hematopoietic-generating properties, HSCs and hematopoietic progenitor cells have been widely used as autologous or allogeneic transplants to treat patients with blood disorders and hematopoietic malignancies.7, 8 However, following transplantation, patients may remain cytopenic and immune defective, with accrued mortality risk arising from engraftment failure, opportunistic infection, and disease relapse.9, 10
During the last decade, remarkable progress has been made in developing new tools to manipulate HSCs and hematopoietic progenitor cells for clinic use. G-CSF (granulocyte colony-stimulating factor), GM-CSF (granulocyte macrophage colony-stimulating factor), and CXCR4 (C-X-C chemokine receptor type 4) antagonists have been utilized to mobilize HSC migration from bone marrow to the blood and increase the number of myeloid progenitors.11, 12 Although these agents can mobilize HSCs to facilitate the harvest of the cells from peripheral blood, they do not lead to expansion of endogenous self-renewing HSCs. An integrated strategy by automated control of inhibitory feedback signaling can expand HSCs ex vivo.13 Using alexidine dihydrochloride and metformin to reprogram HSC metabolism or inhibiting retinoic acid signaling can facilitate the maintenance of HSCs in vitro and promote their self-renewal.14, 15 Notch, Wnt/β-catenin, Bmi-1 (B lymphoma Mo-MLV insertion region 1 homolog), HoxB4 (homeobox B4), and aryl hydrocarbon receptor antagonists have all been shown to promote the expansion of HSCs and progenitor cells in vitro.16, 17, 18, 19, 20 However, in vitro-expanded HSCs have decreased engraftment capacity.21, 22 Until now, there has been no effective method to expand endogenous HSCs in vivo for repopulation of hematopoietic cells and immune system.
GM-CSF and interleukin (IL) common gamma chain cytokine-based fusion genes (GIFT fusokines) have been shown to have novel biological functions distinct from their parental molecules,23, 24 inducing regulatory B cells,25 tumor-killing dendritic cells,26 and natural killer cells.27 Recently, we generated a synthetic fusokine that is derived from the fusion of GM-CSF and IL-4 (named as GIFT4) and found that GIFT4 protein has a potent immune stimulatory property and educates naive B cells into B effector cells with anti-tumor activity.28 Unexpectedly, we discovered that GIFT4 triggers a novel B cell function on hematopoiesis in vivo. Here, we show that GIFT4 treatment expands endogenous LSK cells and its subpopulations, HSCs and multipotent progenitors (MPPs), in a B cell-dependent manner and robustly accelerates long-term hematopoietic reconstitution following myeloablative therapy in mice.
Results
GIFT4 Induces the Endogenous Expansion of Bone Marrow LSK Cells
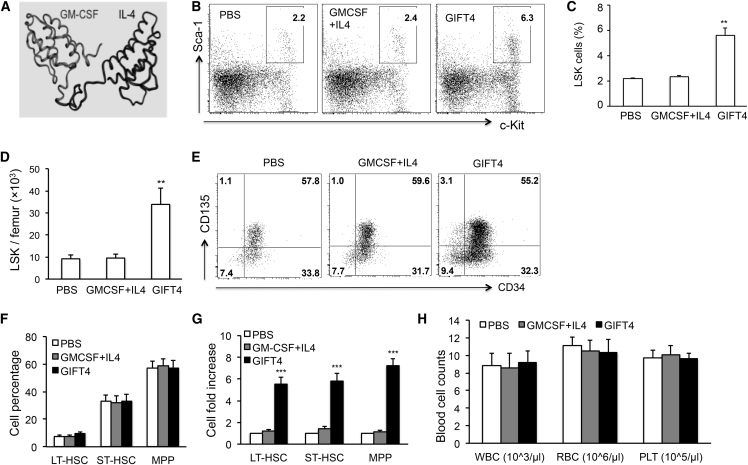
We previously demonstrated that GIFT4, a fusion protein from GM-CSF and IL-4 with a single polypeptide chain of 282 amino acids (Figure 1A), has a potent immune stimulatory function on B cells.28 Surprisingly, delivery of GIFT4 protein into naive C57BL/6J (B6) mice significantly increased bone marrow cellularity (Figure S1) and the proportion of endogenous LSK cells in the Lin-negative population of bone marrow cells in comparison with GM-CSF and IL-4 treatment or PBS controls, as determined by fluorescence-activated cell sorting (FACS) assay (Figure 1B). The percentage of LSK cells in the femur of GIFT4-treated mice was 5.8% on average, 2.5-fold higher than the ones in GM-CSF and IL-4- or PBS-treated mice (2.3% and 2.2% on average, respectively) (Figure 1C). There was no significant difference in LSK cell percentage between the two control groups (Figures 1B and 1C). In comparison with mice treated with GM-CSF and IL-4 or PBS, GIFT4-treated mice had a significant increase in the absolute number of LSK cells (Figure 1D), with an average of 34,000 LSK cells per femur, which is 3.5-fold higher than in control mice. LSK cells are enriched by HSCs and hematopoietic progenitor cells that have the ability to repopulate the hematopoietic system. LSK cells contain three subsets, including long-term HSCs (LT-HSCs) (CD34−CD135− LSK cells), short-term HSCs (ST-HSCs) (CD34+CD135− LSK cells), and multipotent progenitors (MPPs) (CD34+CD135+ LSK cells).4 Analyses of bone marrow LSK subpopulations by FACS demonstrated that the proportions of LT-HSCs, ST-HSCs, and MPPs in LSK cells in mice treated with GIFT4, GM-CSF and IL-4, or PBS were similar (Figures 1E and 1F). However, GIFT4-treated mice had more than a 5-fold increase of LT-HSC, ST-HSC, or MPP cell numbers per femur in comparison with the PBS-treated group (Figure 1G). There was no significant difference in the cell number of LT-HSCs, ST-HSCs, or MPPs in mice treated with GM-CSF and IL-4 or PBS (Figure 1G). Peripheral blood counts showed that GIFT4-treated mice had similar peripheral blood profiles on the compartments of white blood cells, red blood cells, and platelets with GM-CSF and IL-4- or PBS-treated mice or naive mice (Figure 1H). Ki67 is a cell proliferation marker and has been utilized to analyze the cell cycle status of HSCs.29 To determine whether GIFT4 stimulation could trigger the proliferation of bone marrow HSCs and MPPs in vivo, we performed intracellular staining of Ki67 on bone marrow cells isolated from GIFT4-treated mice. FACS analyses revealed that GIFT4 treatment markedly promoted the proliferation of endogenous bone marrow LT-HSCs, ST-HSCs, and MPPs in the treated mice in comparison with GM-CSF and IL-4 or PBS treatment (Figures S2A and S2B). In contrast, there were no Ki67+ LT-HSCs and a very low percentage of Ki67+ ST-HSCs or MPPs in mice treated with GM-CSF and IL-4 or PBS (Figures S2A and S2B). Annexin-V staining of LSK cells showed that GIFT4 treatment also led to a higher percentage of apoptotic death in LT-HSCs compared with ST-HSCs and MPPs (Figures S2A and S2C).
Figure 1.
GIFT4-Triggered Expansion of Endogenous LSK Cells
(A) Predicted 3D structure of murine GIFT4 protein. (B) Naive B6 mice (n = 10 per group) were administrated with GIFT4 protein, GM-CSF and IL-4, or PBS for 6 days. On day 7, bone marrow cells were harvested from the femur of the treated mice and subjected to FACS analyses. Data were representatives of LSK cells gated on Lin− Sca-1+c-Kit+. (C and D) Percentage of LSK cells in Lin− population (C) and absolute number of LSK cells per femur (D) were calculated. (E and F) LSK cells in the three groups of treatments were further profiled with anti-mouse CD34 and CD135 antibodies by FACS (E), and LT-HSC, ST-HSC, and MPP subsets in the LSK compartment were gated on CD34−CD135−, CD34+CD135−, or CD34+CD135+, respectively (F). (G) The cell-number fold change of LT-HSCs, ST-HSCs, or MPPs per femur in GIFT4- or GM-CSF and IL-4-treated mice was calculated based on the PBS-treated group. (H) Peripheral white blood cells (WBC), red blood cells (RBC), and platelets (PLT) per microliter of peripheral blood were also counted on an automatic blood counter. Data were from three independent experiments.
B Cells Are Essential for GIFT4-Induced LSK Expansion In Vivo
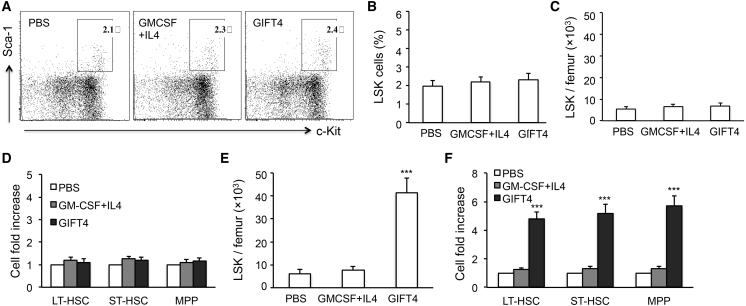
To test our hypothesis that B cells may play an important role in GIFT4-augmented hematopoietic regeneration, we utilized B cell-deficient μ-chain mutant transgenic (μMT) mice that lacked functional B cells but had normal T cell and other immune cellular compartments. μMT mice were treated with GIFT4 protein, control cytokines GM-CSF and IL-4, or PBS. Analysis of LSK cells in the bone marrow of treated mice by FACS showed that there was no significant difference in the LSK cell percentage in the Lin− population among the three groups of treated mice, and GIFT4 treatment could not promote the expansion of endogenous LSK cells in the absence of B cells (Figures 2A and 2B). The absolute number of bone marrow LSK cells per femur was similar among the three groups of mice treated with or without GIFT4 protein (Figure 2C). To examine whether B cells are required for GIFT4-triggered expansion of HSCs and MPPs, we analyzed the three components of LSK cells in the treated μMT mice. The profile of LSK cell subsets in GIFT4-treated mice demonstrated that GIFT4 stimulation could not increase endogenous LT-HSCs, ST-HSCs, and MPPs in the absence of functional B cells (Figure 2D). To test whether the adoptive transfer of B cells could facilitate LSK cell expansion in the bone marrow of μMT mice upon GIFT4 stimulation, we purified splenic B cells from naive B6 mice with a B cell-negative selection kit with the addition of anti-mouse Sca1 and c-Kit antibodies to remove LSK cells in purified B cells. μMT mice that received purified B cells were then treated with GIFT4, GM-CSF and IL-4, or PBS. The profile of LSK cells in the three groups of treated mice demonstrated that GIFT4 treatment significantly increased bone marrow LSK cells in the μMT mice adoptively transferred with naive B cells, which was five times higher than in the mice treated with GM-CSF and IL-4 or PBS (Figure 2E). Consistently, administration of GIFT4 protein significantly increased the number of LT-HSCs, ST-HSCs, and MPPs in B cell-transferred μMT mice in comparison with GM-CSF and IL-4- or PBS-treated μMT mice (Figure 2F). To examine the hematopoietic capability of GIFT4-triggered LSK cells, lethally irradiated B6 mice were transplanted with LSK cells (Figure S3A). Blood counting and mice body weight change demonstrated that mice that received GIFT4-augmented LSK cells had similar hematopoietic regeneration (Figure S3B) and body weight recovery (Figure S3C) and survived (Figure S3D), in comparison with mice transferred with normal LSK cells (Figures S3B–S3D). PBS-treated mice died from hematopoietic failure within 2 weeks after irradiation (Figures S3B and S3D).
Figure 2.
Role of B Cells in GIFT4-Induced LSK Expansion
B cell-deficient μMT mice (n = 10 per group) were treated with GIFT4, GM-CSF and IL-4, or PBS for 6 days. Bone marrow cells were then harvested from the femur for FACS assay with anti-mouse Lin, Sca-1, c-Kit, CD34, and CD135 antibodies. (A) Data were representatives of LSK cells gated on the Lin−Sca-1+c-Kit+ population. (B and C) Percentage of LSK cells in Lin-negative population (B) and absolute number of LSK cells per femur (C) in the three groups of mice were calculated and presented. (D) The cell-number fold change of LT-HSCs, ST-HSCs, or MPPs per femur in GIFT4- or GM-CSF and IL-4-treated mice was calculated based on the PBS-treated group. (E and F) Alternatively, B cells purified from naive B6 mice were adoptively transferred into μMT mice. The mice were then treated with GIFT4, GM-CSF and IL-4, or PBS for 6 days. The number of LSK cells per femur in the treated mice was quantified by FACS with anti-Lin, c-Kit, and Sca-1 antibodies (E), and the cell-number fold increase of LT-HSCs, ST-HSCs, or MPPs per femur in GIFT4- or GM-CSF and IL-4-treated mice was calculated based on the PBS control group (F). Data were from three independent experiments.
Blocking B Cell-Derived Leukines Suppresses GIFT4-Induced HSC Expansion
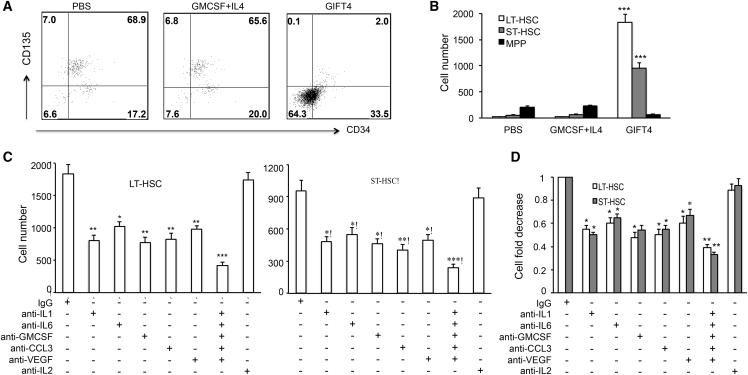
We previously showed that GIFT4-treated B cells (GIFT4-B cells) produce a panel of leukines, including IL-1, IL-6, GM-CSF, CCL3 (C-C motif chemokine ligand 3), and VEGF (vascular endothelial growth factor). To test whether GIFT4 treatment could increase bone marrow LT-HSCs, ST-HSCs, and MPPs in vitro and whether those leukines contribute to the augmentation of HSCs and MPPs, we stimulated bone marrow cells with GIFT4, GM-CSF and IL-4, or PBS for 4 days or with GIFT4 protein in the presence or absence of specific neutralizing antibodies against those leukines. The profile of the three LSK components by FACS demonstrated that GIFT4 treatment significantly increased the percentage and absolute cell number of LT-HSCs or ST-HSCs in the cell culture system in comparison with GM-CSF and IL-4 or PBS treatment (Figures 3A and 3B). There were few MPPs in GIFT4-treated bone marrow cells (Figure 3B). Blocking the activities of IL-1, IL-6, GM-CSF, CCL3, or VEGF by the addition of specific neutralizing antibodies in the GIFT4 and bone marrow cell culture system significantly reduced the number of LT-HSCs and ST-HSCs in the culture (Figure 3C), although neutralizing antibodies had no significant effect on the proportion of HSCs and MPPs in the LSK compartment (Figure S4). Interestingly, the combination of those neutralizing antibodies further suppressed HSCs in comparison with individual antibody (Figure 3C). However, anti-mouse IL-2-neutralizing antibodies had no suppressive effect on GIFT4-mediated HSC augmentation (Figure 3C). To further examine whether neutralizing the activity of GIFT4-B cell-secreted cytokines could have a suppressive effect on HSCs, GIFT4-B cells were co-cultured with sorted LSK cells in the presence of these antibodies for 4 days. Indeed, antibodies against IL-1, IL-6, GM-CSF, CCL3, or VEGF, but not IL-2, significantly reduced the number of LT-HSCs or ST-HSCs in the cell culture system in comparison with control immunoglobulin G (IgG) treatment (Figure 3D).
Figure 3.
Contribution of B Cell-Derived Leukines to GIFT4-Augmented HSCs
Bone marrow cells isolated from B6 mice were treated with GIFT4, GM-CSF and IL-4, or PBS for 4 days and subjected to FACS analyses with anti-mouse Lin, Sca-1, c-Kit, CD34, and CD135 antibodies. (A) Data were representatives of LSK subset LT-HSCs, ST-HSCs, and MPPs gated on CD34−CD135−, CD34+CD135−, and CD34+CD135+, respectively. (B) Absolute cell number of LT-HSCs, ST-HSCs, or MPP, in the three groups of cell culture with GIFT4, GM-CSF and IL-4, or PBS were calculated and presented. (C) Bone marrow cells were treated with GIFT4 protein in the presence of anti-mouse IL-1-, IL-6-, GM-CSF-, CCL3-, and VEGF-specific neutralizing antibodies or combined antibodies, anti-mouse IL-2-neutralizing antibody, or control IgG for 4 days before they were subjected to FACS analyses. (D) Alternatively, LSK cells were co-cultured with GIFT4-B cells in the presence of these neutralizing antibodies or control IgG for 4 days. The absolute cell number of LT-HSCs or ST-HSCs (C) or the cell-number fold decrease in comparison with IgG control treatment (D) in the culture was calculated and presented. Data were from three independent experiments.
Administration of GIFT4 and B Cells Augments Hematopoietic Regeneration
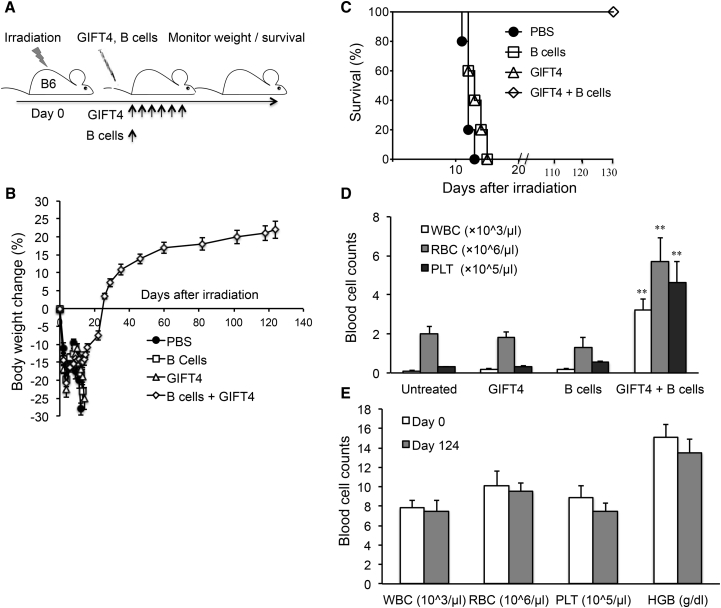
To test whether GIFT4 protein could serve on its own as a hematopoietic augmenter to restore the blood system after lethal irradiation, we radiated B6 mice with a dose of 11Gy and treated mice with GIFT4 protein or purified B cells only or GIFT4 protein plus B cells or PBS (Figure 4A). Mice did not receive any bone marrow or LSK cells as hematopoietic rescue. Monitoring murine body weight demonstrated that the delivery of GIFT4 protein plus B cells into radiated mice promoted body weight recovery on day 124 after irradiation (Figure 4B) and long-term survival (Figure 4C). In contrast, mice that received GIFT4, B cells only, or PBS continued to lose weight (Figure 4B) and died from bone marrow failure within 2 weeks after irradiation (Figure 4C). Peripheral blood counting showed that the delivery of GIFT4 protein to the mice did not prevent pancytopenia caused by lethal irradiation, and mice had a very low number of blood cells on day 13 after irradiation, similar to the mice treated with B cells only or PBS (Figure 4D). However, mice administrated with GIFT4 protein and naive B cells had a significantly higher number of blood cells in the circulation on day 13, compared to the ones treated with GIFT4 protein or B cells alone or PBS (Figure 4D). On day 124 after irradiation, mice treated with GIFT4 plus B cells had almost complete hematopoietic regeneration, with similar levels of white blood cells, red blood cells, platelets, and hemoglobin in the circulation as the baseline before irradiation (Figure 4E). Adoptive transfer of LSK cells from GIFT4 and B cell-treated mice into naive irradiated B6 mice further confirmed its hematopoietic capability similar to LSK cells isolated from naive B6 mice (Figure S5).
Figure 4.
Hematopoietic Regeneration Induced by GIFT4 and B Cell Treatment
(A) Naive B6 mice (n = 10 per group) were radiated with a 11Gy dose and treated with GIFT4 protein, splenic B cells only (from B6 mice), PBS, or a combination of GIFT4 and B cells by intravenous injection on the next day. Delivery of GIFT4 protein lasted for 6 days. (B and C) Mouse body weight change (B) and survival (C) were monitored and calculated. (D) Blood cell profiles per microliter peripheral blood in three groups of mice were analyzed with a blood counter on day 13 after irradiation. (E) On day 124 after irradiation, peripheral blood cellular components per microliter peripheral blood or hemoglobin level in mice treated with GIFT4 and B cell were profiled with the blood counter in comparison with the base lines on day 0 before irradiation. Data were from three independent experiments.
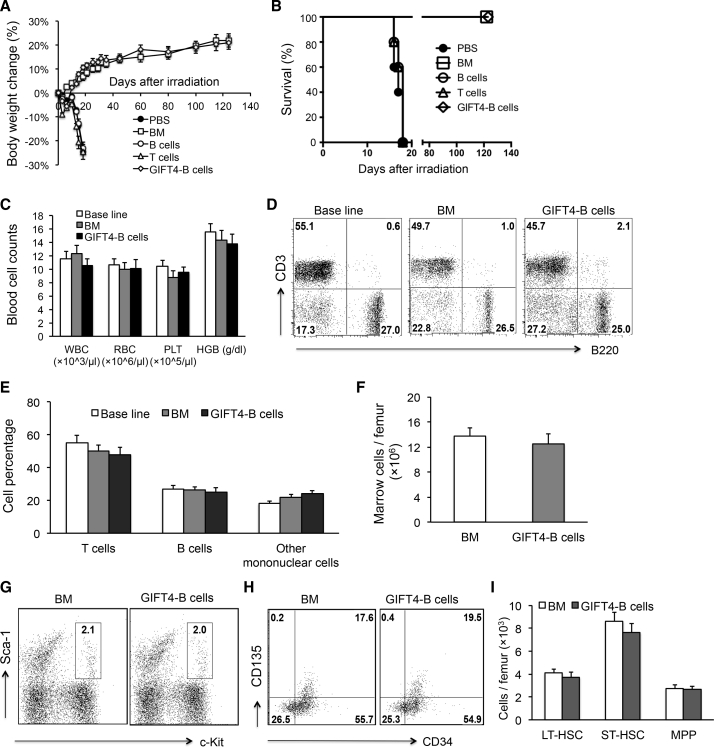
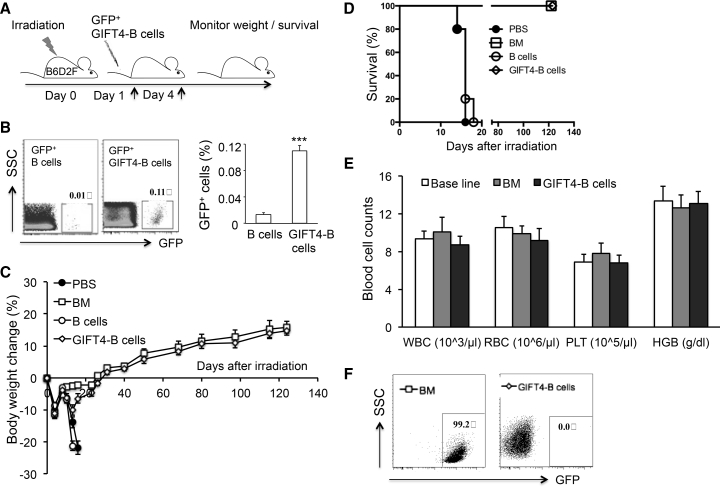
GIFT4-B Cells Enable Hematopoietic Recovery in Irradiated Mice
To explore the potential of GIFT4-B cells as an autologous cell therapy, we produced GIFT4-B cells in vitro and adoptively transferred the cells or syngeneic bone marrow cells, naive B cells (Figure S6A), or T cells into radiated mice (11Gy) on day 1 and day 4. GIFT4-B cells, as well as purified naive B cells, are absent of LSK cells (Figure S6B) and express a panel of B effector markers (Figure S6C), as we previously showed.28 GFP-positive GIFT4-B cells have the capability to migrate into the bone marrow and spleen, but not the brain, in vivo (Figures S6C and S6D). The measurement of body weight showed that the delivery of GIFT4-B cells prevented mice from irradiation-induced body weight loss (Figure 5A); the mice continued to gain weight and survived similarly to bone marrow-transferred mice (Figure 5B). However, control mice that received normal B cells, T cells, or PBS treatment failed to recover from body weight loss and died (Figures 5A and 5B). Peripheral blood counts on day 124 after irradiation further confirmed that GIFT4-B cell treatment completely restored hematopoiesis in mice (Figure 5C). Analyses of T cell and B cell components in peripheral blood in mice treated with GIFT4-B cells or bone marrow cells on day 124 after irradiation demonstrated that GIFT4-B cell treatment promoted T cell and B cell recovery close to the one in bone marrow-transplanted mice or the baseline level before irradiation (Figures 5D and 5E). Bone marrow cellularity in mice that received GIFT4-B cells and bone marrow cells were similar (Figure 5F). There was also no significant difference in the profiles of LSK cells (Figure 5G) and the three subsets between GIFT4-B cell-treated mice and bone marrow-transferred mice (Figures 5H and 5I). Transplantation of LSK cells from GIFT4-B cell-treated mice into irradiated B6 mice further confirmed the hematopoietic ability of the LSK cells similar to the ones isolated from naive mice (Figure S5).
Figure 5.
GIFT4-B Cell Augmented Hematopoietic Regeneration in Murine Irradiation-Induced Bone Marrow Failure Model
(A) Irradiated (11Gy) naive B6 mice (n = 10 per group) were transplanted with syngeneic GIFT4-B cells, bone marrow cells, or purified splenic B cells, T cells (from B6 mice), or PBS on day 1 and day 4 after irradiation by intravenous injection. Mouse body weight was measured with a digital scale, and weight change percentage was calculated. (B) Mice death in the three groups after irradiation was monitored, and the survival percentage was calculated. (C) Peripheral blood in mice treated with GIFT4-B cells or bone marrow cells was harvested from tail veins on day 124 after irradiation, and the numbers of white blood cells (WBC), red blood cells (RBC), and platelets (PLT) per microliter of peripheral blood and hemoglobin levels were profiled on an automatic blood counter in comparison with the baseline levels on day 0 before irradiation. (D and E) T cell and B cell components in peripheral blood harvested from the mice on day 0 before irradiation or from the irradiated mice received GIFT4-B cell or bone marrow cell treatment on day 124 were profiled by FACS (D), and the percentage of T cells, B cells, or other mononuclear cells (CD3−B220− cells) was calculated and presented (E). (F) Bone marrow cells in mice treated with bone marrow cells or GIFT4-B cells were harvested on day 124 after irradiation, and bone marrow cellularity was presented with marrow cells per femur. (G–I) LSK cells and its three subsets, LT-HSC, ST-HSC, and MPP, in bone marrow were gated on Lin−Sca-1+c-Kit+ (G), Lin−Sca-1+c-Kit+CD34−CD135−, Lin−Sca-1+c-Kit+CD34+CD135−, and Lin−Sca1+c-Kit+CD34+CD135+ (H), respectively, and the absolute number (per femur) of LT-HSCs, ST-HSCs, and MPPs was calculated and presented (I). Data were from three independent experiments.
To test whether allogeneic GIFT4-B cells could function similarly as autologous GIFT4-B cells in hematopoiesis, we generated GIFT4-B cells from GFP-positive B6 mice, and adoptively transferred the GFP+ GIFT4-B cells into B6D2F1/J mice (allogeneic to B6 mice)30 as stated above (Figure 6A). FACS analysis showed that seven times more GFP+ GIFT4-B cells than GFP+ naive B cells entered into the bone marrow within 48 hr after intravenous injection (Figure 6B). We also transferred GFP-positive bone marrow cells isolated from GFP+ mice into radiated mice serving as positive control treatment. Measurement of mouse body weight showed that mice receiving allogeneic GIFT4-B cells prevented mice from losing weight after irradiation, which was slightly lower than mice that received GFP+ bone marrow cells. Both groups of mice survived lethal irradiation for a long-term period (124 days) (Figures 6C and 6D). Consistently, PBS-treated mice or mice transferred with normal B cells failed to gain weight and died (Figures 6C and 6D). Peripheral blood counting showed that allogeneic GIFT4-B cell-treated mice had similar levels of white blood cells, red blood cells, platelets, and hemoglobin levels as bone marrow-transplanted mice on day 124 after irradiation, which recovered to the baseline level before irradiation (Figure 6E). FACS analysis on peripheral blood further showed that white blood cells in mice that received GFP+ bone marrow were 99.2% GFP positive (Figure 6F), confirming hematopoietic recovery came from donor LSK cells. However, white blood cells in mice adoptively transferred with allogeneic GFP+ GIFT4-B cells were 99.9% GFP negative (Figure 6F), demonstrating that long-term hematopoietic regeneration after lethal irradiation was derived from endogenous autologous HSCs.
Figure 6.
Hematopoietic Recovery Enhanced by Allogeneic GIFT4-B Cells
(A) Naive B6D2F1/J mice (n = 10 per group) were irradiated with a dose of 11Gy and treated with GFP-positive allogeneic GIFT4-B cells, B cells (B6 background), or PBS on day 1 and day 4 after irradiation or received GFP+ bone marrow cells on day 1. (B) Bone marrow cells were harvested from irradiated mice (n = 5) 48 hr after intravenous injection of GFP+ GIFT4-B cells or GFP+ naive B cells and subjected to FACS analysis. The percentage of exogenous B cells migrated into bone marrow were gated on GFP+ cells. (C and D) Mouse body weight change (C) and survival (D) in the four groups of mice were monitored and calculated. (E) Peripheral blood cell profiles per microliter of peripheral blood in allogeneic GFP+ bone marrow cell- or GFP+ GIFT4-B cell-treated mice were analyzed with a blood counter on day 124 after irradiation, compared with the base lines on day 0 before irradiation. (F) GFP+ white blood cells in the peripheral blood of mice that received GFP+ bone marrow cells or GFP+ GIFT4-B cells were profiled by FACS on day 124 after irradiation.
Discussion
In this study, we demonstrated that GIFT4 fusokine induces B cell-mediated expansion of endogenous LSK cells in bone marrow and GIFT4-B cells promote hematopoietic regeneration in a murine model of irradiation-induced hematopoietic failure. To our knowledge, it is the first report that an engineered cytokine triggers a previously undescribed B cell function on hematopoiesis, which supports the new concept that B cells are involved in the process of hematopoietic regeneration.
The bone marrow niche consists of stromal cells, endothelial cells, osteoblasts, B cells, and other immune cells,31, 32, 33 which regulate the proliferation, differentiation, and mobilization of HSCs.31, 32 As a heterogeneous population in bone marrow microenvironment,34 B cells have been shown to act as the main osteoprotegerin producer in bone marrow for maintaining bone basal homeostasis in vivo35 and play a role in the mobilization of HSCs.36 However, whether B cells play a role in hematopoiesis or hematopoietic regeneration remains unclear. In the clinic, anti-CD20 antibody rituximab therapy to deplete B cells for the treatment of patients with B cell lymphoma and autoimmune diseases often causes white cell maturation arrest, the lack of granulocyte progenitors, and hypocellularity in the bone marrow niche,37, 38 accompanied by life-threatening cytopenias,39 suggesting a potential role of B cells in the steady state of hematopoiesis. In this study, our data demonstrated that GM-CSF and IL-4-derived GIFT4 fusokine induces robust expansion of endogenous LSK cells, including HSCs and MPPs, through GIFT4-activated B cells. The non-responsiveness of LSK cells to GIFT4 stimulation in the absence of B cells in B cell-deficient μMT mice and the GIFT4-induced expansion of LSK cells in μMT mice requiring exogenous naive B cells strongly indicate that B cells have a stimulatory function on hematopoietic stem and progenitor cells. Consistently, without exogenous B cells, GIFT4 protein alone could not augment hematopoiesis in a murine model of radiation-induced hematopoietic failure, further informing an entirely novel function of B cells on hematopoietic regeneration. In a preclinical radiation-induced bone marrow failure mouse model, adoptive transfer of syngeneic or allogeneic GIFT4-B cells augments long-term survival and complete hematopoietic recovery in lethally irradiated mice, highlighting the impact of B cells in the process of hematopoietic regeneration.
Cytokines, chemokines, and growth factors, such as IL-1, IL-3, IL-6, stem cell factor (SCF), GM-CSF, G-CSF, and VEGF produced by the cellular components in the bone marrow stem cell niche contribute to HSC self-renewal, expansion, and differentiation. G-CSF has been shown to activate dormant HSCs into self-renewal.40 IL-6 has be demonstrated to have a synergetic effect with other cytokines and growth factors, including IL-3 and SCF, to expand HSCs in vitro or ex vivo.41, 42 A single-cell proteomics platform showed that IL-6 is particularly important for regulating myeloid differentiation and the proliferation of hematopoietic stem and progenitor cells; neutralizing IL-6 or GM-CSF reduces the number of LSK cells.43 VEGF is required for adult HSC formation and survival44 and is essential for erythropoiesis.45 Murine GIFT4-B cells produce an array of hematopoietic cytokines and growth factors, including IL-1, IL-6, GM-CSF, VEGF, and chemokine CCL3, but not G-CSF and SCF.28 The role of CCL3 on hematopoiesis is controversial. In the presence of GM-CSF or M-CSF or in combination with SCF, IL-3, IL-6, and Flt3L, CCL3 has a stimulatory effect on the expansion and proliferation of hematopoietic progenitor cells.46, 47 However, CCL3 together with transforming growth factor β (TGF-β), CCL2, CCL4, and CXCL10 showed an inhibitory effect on HSC expansion.13 Our data demonstrate that inhibiting the activities of IL-1, IL-6, GM-CSF, CCL3, or VEGF by neutralizing antibodies significantly reduced GIFT4- and GIFT4-B cell-augmented LT-HSCs and ST-HSCs in vitro, suggesting that the aggregate pool of pro-hematopoietic secretome, consisting of IL-1, IL-6, VEGF, GM-CSF, and CCL3 from GIFT4-B cells, could contribute to GIFT4-induced B cell-dependent expansion of HSCs in vivo.
GIFT4-B cells possess unique secretion of pro-hematopoietic growth factors, cytokines, and chemokines.28 It was reported that GM-CSF promoted hematopoietic recovery by enhancing the survival of HSCs and the proliferation and differentiation of myeloid lineage cells in irradiated mice.48, 49 Cytokines IL-1 and IL-12 also have pro-hematopoietic effects, enhancing hematopoiesis and immune reconstitution following irradiation.50, 51, 52, 53 Chemokine CCL3 has been shown to help hematopoietic recovery after several cycles of radiation therapy by maintaining the self-renewal capacity of HSCs.54 Growth factor VEGF was reported to have mitogenic activity to suppress HSC apoptotic death and play a role in the survival and maintenance of HSCs.55 Indeed, the delivery of autologous or allogeneic GIFT4-B cells rescued the irradiated mice from hematopoietic injury and augmented hematopoietic regeneration and long-term survival. By blocking the activities of GIFT4-B cell secretome with specific neutralizing antibodies abrogated the pro-hematopoietic function of GIFT4-B cells on HSCs. Conversely, naive B cells or GM-CSF and IL-4-treated B cells do not produce IL-1, IL-6, VEGF, GM-CSF, and CCL3,28 and the delivery of naive B cells or B cells plus GM-CSF and IL-4 treatment could not induce the expansion of LSK cells in B cell-deficient mice and failed to repopulate hematopoietic system in irradiated mice, further indicating that GIFT4-B cells have a unique pro-hematopoietic activity.
In summary, our study has identified a previously undescribed novel B cell function that supports hematopoiesis and hematopoietic regeneration. Based on the activities of GIFT4 protein and GIFT4-B cells in tumor28 and hematopoietic regeneration, we propose that GIFT4 and GIFT4-B cells could serve as means to augment long-term hematopoietic engraftment in the setting of autologous and allogeneic bone marrow transplantation for patients with hematological malignancy.
Materials and Methods
GIFT4 Protein
GIFT4 chimeric transgene was cloned from murine GM-CSF and IL-4 cDNA (Invivogen), and the fusion protein was produced in bio-engineered 293T cells as previously described.28 Protein was quantified by an ELISA kit with anti-murine GM-CSF antibodies (eBiosciences).
Cell Culture
Bioengineered 293T cells stably expressing murine GIFT4 protein were cultured in DMEM supplemented with 10% fetal bovine serum. Murine splenic B cells from B6 or GFP-positive transgenic mice (B6 background) were purified by negative selection with pan B cell enrichment kit (StemCell) with the addition of biotin-conjugated anti-mouse c-Kit and Sca-1 antibodies (1:50 dilution) (Biolegend). Purified B cells were cultured in complete RPMI 1640 medium (5 × 105 cells/mL) in the presence of GIFT4, GM-CSF, and IL-4 (2 ng/mL) (PeproTech) for 5 days. The cells were then harvested for in vivo experiments. Bone marrow cells (5 × 105 cells/mL) harvested from naive B6 mice were treated with GIFT4 protein, GM-CSF, and IL-4 (2 ng/mL) or PBS for 4 days in complete RPMI 1640 medium. Bone marrow cells (5 × 105 cells/mL) were cultured with GIFT4 protein (2 ng/mL) in the presence of anti-mouse IL-1-, IL-2-, IL-6-, GM-CSF-, CCL3-, or VEGF-specific neutralizing antibodies (5 μg/mL) or control IgG isotype (PeproTech) for 4 days. Alternatively, sorted bone marrow LSK cells (104 cells/mL) from naive B6 mice were co-cultured with GIFT4-B cells (105 cells/mL) in the presence of cytokine-neutralizing antibodies or control IgG for 4 days before they were subjected to FACS analyses.
Flow Cytometry
Bone marrow cells were harvested from the in vitro cell culture system or the femur of B6 mice, B cell-deficient μMT mice (B6.129S2-Igh-6tm1Cgn/J), or B6D2F1/J mice (Allogeneic to B6) and were stained with peridinin chlorophyll (PerCP)-conjugated anti-mouse Sca-1 allophycocyanin (APC)-conjugated anti-mouse c-Kit, biotin-labeled anti-lineage antibodies (CD2, CD3, CD4, CD8, CD11b, CD11c, CD14, B220, NK1.1, Gr-1, and TER-119) (Biolegend), fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD34, and anti-mouse CD135 (Brilliant Violet 421) for 30 min at 4°C and then reacted with phycoerythrin (PE)-conjugated streptavidin. After a wash with PBS, the cells were subjected to FACS analyses on a BD Canto Flow Cytometer. Alternatively, bone marrow cells harvested from B6 mice were subjected to Annexin-V staining or intracellular staining with PE-conjugated anti-Ki67 antibody (BD) after surface staining with anti-mouse Sca-1, CD117, CD34, and CD135 antibodies. LSK cells were also sorted from the Lin−Sca1+c-Kit+ cell population in the bone marrow isolated from naive- or GIFT4-treated B6 mice or irradiated mice on a BD Aria Cell Sorter for in vitro or in vivo experiments. For determining the initiation of hematopoietic recovery from endogenous or exogenous LSK cells, irradiated B6D21/J mice were treated with GFP+ GIFT4-B cells or GFP+ bone marrow cells. Peripheral blood cells, splenocytes, and bone marrow cells were harvested from treated mice. After lysis of red blood cells, leukocytes were subjected to FACS analyses with anti-CD3 or B220 antibodies (BD), and GFP-positive cells were also gated. All FACS data were analyzed with FlowJo 9.1 software.
Blood Cell Counting
Peripheral blood was harvested from B6, μMT, or B6D2F1/J mice directly to microvette tubes containing ethylenediaminetetraacetic acid-tripotassium salt (Sarstedt) and subjected to blood cell counting on a Vet-ABC Animal Blood Counter (Scil) using a mouse software provided by the company.
Mouse Experiments
Naive B6 and μMT mice were treated with GIFT4, GM-CSF and IL-4 (20 ng/mouse/day), or PBS by intravenous injection for 6 days (10 mice per group). Femurs were harvested for LSK cell analysis. For mouse total body irradiation to induce bone marrow failure, naive B6 or B6D2F1/J mice were treated with a dose of 11Gy total body irradiation (5.5Gy + 5.5Gy with a 3 hr interval) as previously described.56 Irradiated B6 mice were treated with LSK cells (104 cells/mouse) from naive or GIFT4-treated B6 mice or treated with GIFT4 protein (20 ng/mouse/day), B cells (5 × 106 cells/mouse), or GIFT4 protein together with B cells. PBS treatment served as a negative control. Irradiated B6 or B6D2F1/J mice were transfused with GIFT4-B cells (B6 background), GFP+ GIFT4-B cells (B6 background, allogeneic to B6D2F1/J mice) (5 × 106 cells/mouse/time), GFP+ or wild-type (from B6 mice) bone marrow cells (105 cells/mouse), naive B cells, or T cells (5 × 106 cells/mouse/time). Alternatively, irradiated B6 mice were transfused with LSK cells (104 cells/mouse) from naive or irradiation-survived mice in the setting of GIFT4 plus B cell treatment or GIFT4-B cell treatment. B6, μMT, and B6D2F1/J mice (6–8 weeks old, male) were purchased from Jackson Laboratory. GFP+ transgenic mice57 were provided by Dr. Masaru Okabe. The use of mice and the irradiation of mice were approved by Emory University.
Statistical Analysis
Data were shown as mean ± SEM. p values were calculated using the one-way analysis of variance test. A p value of less than 0.05 was considered significant (*p < 0.05; **p < 0.01; ***p < 0.001).
Author Contributions
Concept and design: J.D., J.G.; Acquisition of data (provided animals, provided facilities, etc.): J.D., Y.L., A.P., Y.S., E.K.W.; Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J.D., J.G.; Writing, review, and/or revision of the manuscript: J.D., J.G.; Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J.D., Y.L., A.P., S.Y.; Other (development of the structural model of fusion protein): J.H.W.
Conflicts of Interest
The authors have no conflicts of interest.
Acknowledgments
This work was supported by NIH grant 5R01AI093881 and the Georgia Cancer Coalition (to J.G.), NIH/NCATS grant UL1TR000454, ACTSI GRA ImmunoEngineering Pilot grant 0000030444, the Winship Myeloma Research Fund (P00044695), and the Developmental Fund of Winship Cancer Center Support grant 5P30CA138292-06 (to J.D.). The authors thank Cynthia Giver and Mojibade Natasha in the Department of Hematology and Medical Oncology at Emory University for their assistance on mice irradiation.
Footnotes
Supplemental Information includes six figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2016.11.013.
Contributor Information
Jiusheng Deng, Email: jdeng2@emory.edu.
Jacques Galipeau, Email: jgalipe@emory.edu.
Supplemental Information
References
- 1.Seita J., Weissman I.L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa M., LaRue A.C., Mehrotra M. Plasticity of hematopoietic stem cells. Best Pract. Res. Clin. Haematol. 2015;28:73–80. doi: 10.1016/j.beha.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Lin K.K., Goodell M.A. Detection of hematopoietic stem cells by flow cytometry. Methods Cell Biol. 2011;103:21–30. doi: 10.1016/B978-0-12-385493-3.00002-4. [DOI] [PubMed] [Google Scholar]
- 4.Yang L., Bryder D., Adolfsson J., Nygren J., Månsson R., Sigvardsson M., Jacobsen S.E. Identification of Lin(-)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 5.Ding L., Morrison S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pronk C.J., Rossi D.J., Månsson R., Attema J.L., Norddahl G.L., Chan C.K., Sigvardsson M., Weissman I.L., Bryder D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Czechowicz A., Weissman I.L. Purified hematopoietic stem cell transplantation: the next generation of blood and immune replacement. Hematol. Oncol. Clin. North Am. 2011;25:75–87. doi: 10.1016/j.hoc.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gajewski J.L., Johnson V.V., Sandler S.G., Sayegh A., Klumpp T.R. A review of transfusion practice before, during, and after hematopoietic progenitor cell transplantation. Blood. 2008;112:3036–3047. doi: 10.1182/blood-2007-10-118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunin N., Small T., Szabolcs P., Baker K.S., Pulsipher M.A., Torgerson T. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: persistent immune deficiency in pediatric transplant survivors. Biol. Blood Marrow Transplant. 2012;18:6–15. doi: 10.1016/j.bbmt.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hourigan C.S., McCarthy P., de Lima M. Back to the future! The evolving role of maintenance therapy after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2014;20:154–163. doi: 10.1016/j.bbmt.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motabi I.H., DiPersio J.F. Advances in stem cell mobilization. Blood Rev. 2012;26:267–278. doi: 10.1016/j.blre.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Rettig M.P., Ansstas G., DiPersio J.F. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csaszar E., Kirouac D.C., Yu M., Wang W., Qiao W., Cooke M.P., Boitano A.E., Ito C., Zandstra P.W. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10:218–229. doi: 10.1016/j.stem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Liu X., Zheng H., Yu W.M., Cooper T.M., Bunting K.D., Qu C.K. Maintenance of mouse hematopoietic stem cells ex vivo by reprogramming cellular metabolism. Blood. 2015;125:1562–1565. doi: 10.1182/blood-2014-04-568949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiaur G., Yegnasubramanian S., Perkins B., Gucwa J.L., Gerber J.M., Jones R.J. Regulation of human hematopoietic stem cell self-renewal by the microenvironment’s control of retinoic acid signaling. Proc. Natl. Acad. Sci. USA. 2013;110:16121–16126. doi: 10.1073/pnas.1305937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delaney C., Heimfeld S., Brashem-Stein C., Voorhies H., Manger R.L., Bernstein I.D. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat. Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amsellem S., Pflumio F., Bardinet D., Izac B., Charneau P., Romeo P.H., Dubart-Kupperschmitt A., Fichelson S. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat. Med. 2003;9:1423–1427. doi: 10.1038/nm953. [DOI] [PubMed] [Google Scholar]
- 18.Boitano A.E., Wang J., Romeo R., Bouchez L.C., Parker A.E., Sutton S.E., Walker J.R., Flaveny C.A., Perdew G.H., Denison M.S. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry J.M., He X.C., Sugimura R., Grindley J.C., Haug J.S., Ding S., Li L. Cooperation between both Wnt/beta-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 2011;25:1928–1942. doi: 10.1101/gad.17421911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwama A., Oguro H., Negishi M., Kato Y., Morita Y., Tsukui H., Ema H., Kamijo T., Katoh-Fukui Y., Koseki H. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity. 2004;21:843–851. doi: 10.1016/j.immuni.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Gothot A., van der Loo J.C., Clapp D.W., Srour E.F. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34(+) cells in non-obese diabetic/severe combined immune-deficient mice. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- 22.Glimm H., Oh I.H., Eaves C.J. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0) Blood. 2000;96:4185–4193. [PubMed] [Google Scholar]
- 23.Rafei M., Wu J.H., Annabi B., Lejeune L., François M., Galipeau J. A GMCSF and IL-15 fusokine leads to paradoxical immunosuppression in vivo via asymmetrical JAK/STAT signaling through the IL-15 receptor complex. Blood. 2007;109:2234–2242. doi: 10.1182/blood-2006-07-037473. [DOI] [PubMed] [Google Scholar]
- 24.Ng S., Galipeau J. Concise review: engineering the fusion of cytokines for the modulation of immune cellular responses in cancer and autoimmune disorders. Stem Cells Transl. Med. 2015;4:66–73. doi: 10.5966/sctm.2014-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafei M., Hsieh J., Zehntner S., Li M., Forner K., Birman E., Boivin M.N., Young Y.K., Perreault C., Galipeau J. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat. Med. 2009;15:1038–1045. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]
- 26.Williams P., Bouchentouf M., Rafei M., Romieu-Mourez R., Hsieh J., Boivin M.N., Yuan S., Forner K.A., Birman E., Galipeau J. A dendritic cell population generated by a fusion of GM-CSF and IL-21 induces tumor-antigen-specific immunity. J. Immunol. 2010;185:7358–7366. doi: 10.4049/jimmunol.1002201. [DOI] [PubMed] [Google Scholar]
- 27.Penafuerte C., Bautista-Lopez N., Boulassel M.R., Routy J.P., Galipeau J. The human ortholog of granulocyte macrophage colony-stimulating factor and interleukin-2 fusion protein induces potent ex vivo natural killer cell activation and maturation. Cancer Res. 2009;69:9020–9028. doi: 10.1158/0008-5472.CAN-09-2322. [DOI] [PubMed] [Google Scholar]
- 28.Deng J., Yuan S., Pennati A., Murphy J., Wu J.H., Lawson D., Galipeau J. Engineered fusokine GIFT4 licenses the ability of B cells to trigger a tumoricidal T-cell response. Cancer Res. 2014;74:4133–4144. doi: 10.1158/0008-5472.CAN-14-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szade K., Bukowska-Strakova K., Zukowska M., Jozkowicz A., Dulak J. Analysis of Cell Cycle Status of Murine Hematopoietic Stem Cells. Methods Mol. Biol. 2016;1516:91–99. doi: 10.1007/7651_2016_361. [DOI] [PubMed] [Google Scholar]
- 30.Reddy P., Negrin R., Hill G.R. Mouse models of bone marrow transplantation. Biol. Blood Marrow Transplant. 2008;14(1, Suppl 1):129–135. doi: 10.1016/j.bbmt.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ugarte F., Forsberg E.C. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32:2535–2547. doi: 10.1038/emboj.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riether C., Schürch C.M., Ochsenbein A.F. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. 2015;22:187–198. doi: 10.1038/cdd.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercier F.E., Ragu C., Scadden D.T. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 2011;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsley R.C., Thomas M., Srivastava B., Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Toraldo G., Li A., Yang X., Zhang H., Qian W.P., Weitzmann M.N. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S., Li T., Chen Y., Nie Y., Li C., Liu L., Li Q., Qiu L. Granulocyte Colony-Stimulating Factor Induces Osteoblast Inhibition by B Lymphocytes and Osteoclast Activation by T Lymphocytes during Hematopoietic Stem/Progenitor Cell Mobilization. Biol. Blood Marrow Transplant. 2015;21:1384–1391. doi: 10.1016/j.bbmt.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Terrier B., Ittah M., Tourneur L., Louache F., Soumelis V., Lavie F., Casadevall N., Candon S., Hummel A., Mariette X., Buzyn A. Late-onset neutropenia following rituximab results from a hematopoietic lineage competition due to an excessive BAFF-induced B-cell recovery. Haematologica. 2007;92:e20–e23. doi: 10.3324/haematol.11031. [DOI] [PubMed] [Google Scholar]
- 38.Breuer G.S., Ehrenfeld M., Rosner I., Balbir-Gurman A., Zisman D., Oren S., Paran D. Late-onset neutropenia following rituximab treatment for rheumatologic conditions. Clin. Rheumatol. 2014;33:1337–1340. doi: 10.1007/s10067-014-2562-x. [DOI] [PubMed] [Google Scholar]
- 39.Voog E., Morschhauser F., Solal-Céligny P. Neutropenia in patients treated with rituximab. N. Engl. J. Med. 2003;348:2691–2694. doi: 10.1056/NEJM200306263482620. discussion 2691–2694. [DOI] [PubMed] [Google Scholar]
- 40.Wilson A., Laurenti E., Oser G., van der Wath R.C., Blanco-Bose W., Jaworski M., Offner S., Dunant C.F., Eshkind L., Bockamp E. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 41.Duchez P., Rodriguez L., Chevaleyre J., Lapostolle V., Vlaski M., Brunet de la Grange P., Ivanovic Z. Interleukin-6 enhances the activity of in vivo long-term reconstituting hematopoietic stem cells in “hypoxic-like” expansion cultures ex vivo. Transfusion. 2015;55:2684–2691. doi: 10.1111/trf.13175. [DOI] [PubMed] [Google Scholar]
- 42.Lui W.C., Chan Y.F., Chan L.C., Ng R.K. Cytokine combinations on the potential for ex vivo expansion of murine hematopoietic stem cells. Cytokine. 2014;68:127–132. doi: 10.1016/j.cyto.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J.L., Ma C., O’Connell R.M., Mehta A., DiLoreto R., Heath J.R., Baltimore D. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell. 2014;14:445–459. doi: 10.1016/j.stem.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung A., Ciau-Uitz A., Pinheiro P., Monteiro R., Zuo J., Vyas P., Patient R., Porcher C. Uncoupling VEGFA functions in arteriogenesis and hematopoietic stem cell specification. Dev. Cell. 2013;24:144–158. doi: 10.1016/j.devcel.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehn M., Kertész Z., Cammenga J. Hypoxic induction of vascular endothelial growth factor regulates erythropoiesis but not hematopoietic stem cell function in the fetal liver. Exp. Hematol. 2014;42 doi: 10.1016/j.exphem.2014.08.002. 941–4.e1. [DOI] [PubMed] [Google Scholar]
- 46.Broxmeyer H.E., Sherry B., Cooper S., Lu L., Maze R., Beckmann M.P., Cerami A., Ralph P. Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. Interacting effects involving suppression, synergistic suppression, and blocking of suppression. J. Immunol. 1993;150:3448–3458. [PubMed] [Google Scholar]
- 47.Capmany G., Querol S., Cancelas J.A., García J. Short-term, serum-free, static culture of cord blood-derived CD34+ cells: effects of FLT3-L and MIP-1alpha on in vitro expansion of hematopoietic progenitor cells. Haematologica. 1999;84:675–682. [PubMed] [Google Scholar]
- 48.Katsumoto T.R., Duda J., Kim A., Wardak Z., Dranoff G., Clapp D.W., Shannon K. Granulocyte/macrophage colony-stimulating factor and accessory cells modulate radioprotection by purified hematopoietic cells. J. Exp. Med. 2005;201:853–858. doi: 10.1084/jem.20041504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeves G. Overview of use of G-CSF and GM-CSF in the treatment of acute radiation injury. Health Phys. 2014;106:699–703. doi: 10.1097/HP.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 50.van Os R., Lamont C., Witsell A., Mauch P.M. Radioprotection of bone marrow stem cell subsets by interleukin-1 and kit-ligand: implications for CFU-S as the responsible target cell population. Exp. Hematol. 1997;25:205–210. [PubMed] [Google Scholar]
- 51.Capitano M.L., Nemeth M.J., Mace T.A., Salisbury-Ruf C., Segal B.H., McCarthy P.L., Repasky E.A. Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1-, IL-17-, and G-CSF-dependent manner. Blood. 2012;120:2600–2609. doi: 10.1182/blood-2012-02-409805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen T., Burke K.A., Zhan Y., Wang X., Shibata D., Zhao Y. IL-12 facilitates both the recovery of endogenous hematopoiesis and the engraftment of stem cells after ionizing radiation. Exp. Hematol. 2007;35:203–213. doi: 10.1016/j.exphem.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Basile L.A., Ellefson D., Gluzman-Poltorak Z., Junes-Gill K., Mar V., Mendonca S., Miller J.D., Tom J., Trinh A., Gallaher T.K. HemaMax™, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates. PLoS ONE. 2012;7:e30434. doi: 10.1371/journal.pone.0030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lord B.I., Marshall E., Woolford L.B., Hunter M.G. BB-10010/MIP-1 alpha in vivo maintains haemopoietic recovery following repeated cycles of sublethal irradiation. Br. J. Cancer. 1996;74:1017–1022. doi: 10.1038/bjc.1996.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katoh O., Tauchi H., Kawaishi K., Kimura A., Satow Y. Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Res. 1995;55:5687–5692. [PubMed] [Google Scholar]
- 56.Darlak K.A., Wang Y., Li J.M., Harris W.A., Owens L.M., Waller E.K. Enrichment of IL-12-producing plasmacytoid dendritic cells in donor bone marrow grafts enhances graft-versus-leukemia activity in allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2013;19:1331–1339. doi: 10.1016/j.bbmt.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.