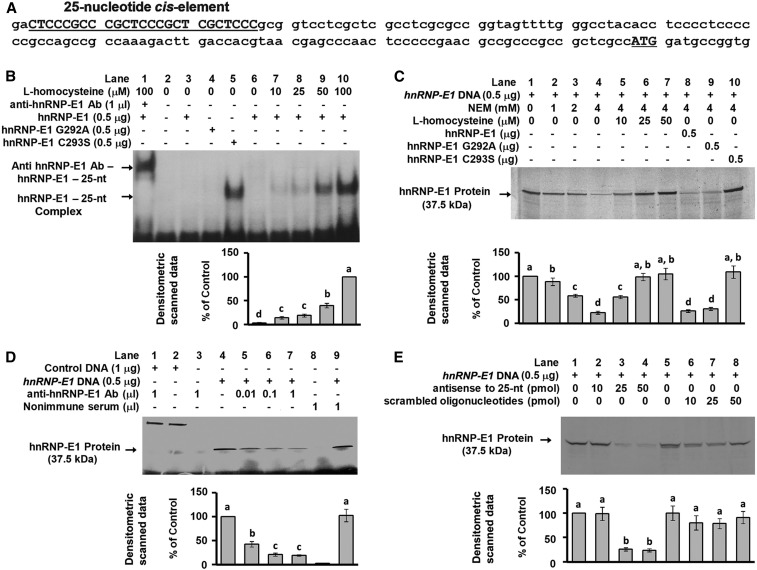
FIGURE 1.
Characterization of the interaction of a 25-nucleotide cis element in the 5′-UTR of hnRNP-E1 with hnRNP-E1 in vitro. Location of the candidate 25-nucleotide cis element (bold, capitalized, and underlined) in the 5′-UTR of hnRNP-E1 from −145 to −120 from the ATG start site (A). Gel-shift analysis of the interaction of radiolabeled 25-nucleotide hnRNP-E1 mRNA cis element and purified recombinant GST–hnRNP-E1 (lanes 1, 3, and 6–10) or hnRNP-E1 mutants (lanes 4 and 5) in the absence or presence of l-homocysteine; supershift of the RNA-protein signal with anti–hnRNP-E1 antiserum (lane 1) (B). In vitro translation of hnRNP-E1 after quenching excess thiols in the reaction mixture by NEM (lane 2–4) to assess the effect of increasing l-homocysteine (lanes 5–7), and comparison of the independent effect of wild-type or mutant hnRNP-E1 (lanes 8–10) (C). Demonstration of the key role of hnRNP-E1 in mediating in vitro translation of hnRNP-E1 protein by the addition of anti–hnRNP-E1 antiserum (lanes 4–7) (D). Lanes 1 and 2 were internal protein controls. Comparison of the effectiveness of antisense oligonucleotides to the 25-nucleotide hnRNP-E1 cis element and scrambled oligonucleotides in quenching the translation of hnRNP-E1 in vitro (E). The autoradiograms shown in B, C, D, and E are representative of 3 separate experiments, and pooled densitometric scanned data of signals are compared with the 100% value. Values are means ± SDs, n = 3 (means of triplicates). Labeled means without a common letter differ, P < 0.05. Ab, antiserum; GST, glutathione S-transferase; hnRNP-E1, wild-type heterogeneous nuclear ribonucleoprotein E1; hnRNP-E1(G292A), wild-type–like heterogeneous nuclear ribonucleoprotein E1 (G292A) mutant; hnRNP-E1(C293S), highest affinity–heterogeneous nuclear ribonucleoprotein E1 (C2923S) mutant; NEM, N-ethylmaleimide; nt, nucleotide; 5′-UTR, 5′-untranslated region.