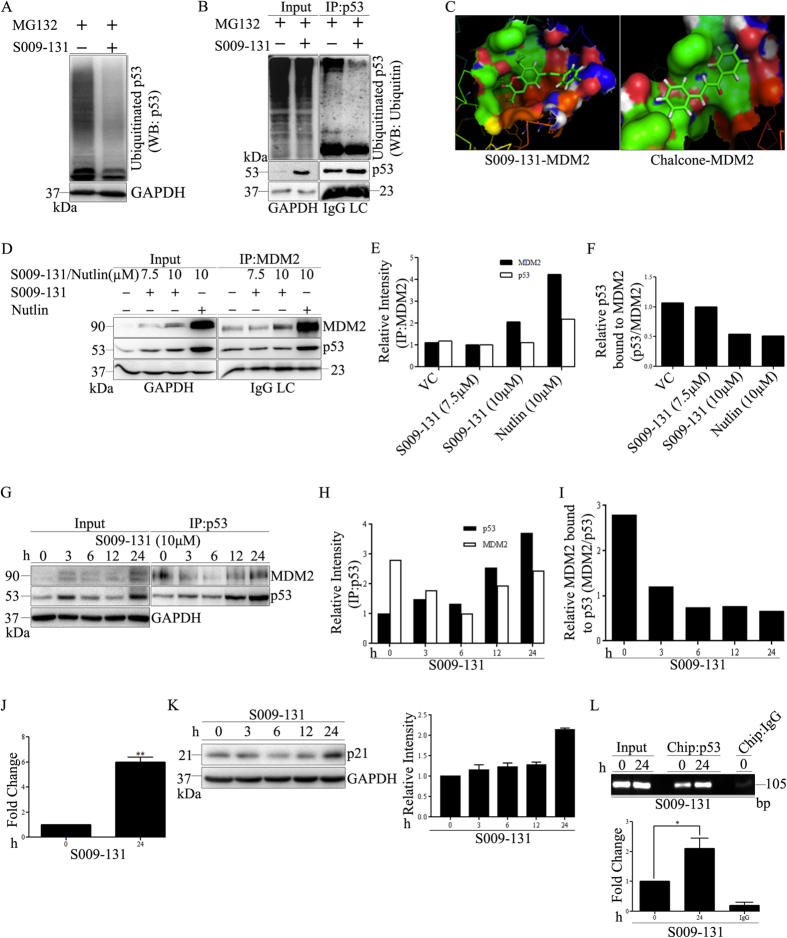
Figure 5. S009-131 protected p53 from proteasomal degradation and enhanced its transcriptional efficiency.
(A) HCT116 cells were co-incubated with 10 μM MG132 and 7.5 μM S009-131. Whole cell lysates were analysed by immunoblotting with anti-p53 antibody. (B) Cell lysates were also subjected to immunoprecipitation (IP) using 1 μg of anti-p53 antibody followed by Western blot for p53 associated ubiquitins. (C) Predicted binding models for S009-131 and chalcone with MDM2. (D) HCT116 cells were treated with S009-131 and Nutlin at indicated concentrations for 24 h. Five hundred micrograms of protein lysates were immunoprecipitated with anti-MDM2 antibody and bound p53 was detected by immunoblotting. (E and F) Band intensities of Co-IP assay were analysed by Image J software and plotted graphically. (G) Western blot assay was performed to show the effect of S009-131 (10 μM) on p53-MDM2 interaction after immuonoprecipitating protein lysates with anti-p53 antibody. (H and I) Band intensities were determined by Image J software and represented graphically. (J) Binding affinity of p53 towards responsive elements (p53-RE) at promoter region before and after S009-131 treatment was determined by co-transfecting cells with EGFP-C1 and p53-Luc Cis Reporter plasmids. Luminescence were normalised by comparing with relative fluorescence in treated and untreated cells. (K) Western blot assay of whole cell lysates from S009-131 treated HCT116 cells to determine p21 protein level. (L) Chromatin immunoprecipitation (ChIP) assay was performed by immunoprecipitating chromatin complex from S009-131 treated and untreated cells with anti-p53 antibody followed by quantitative RT-PCR using p21 promoter specific primers.