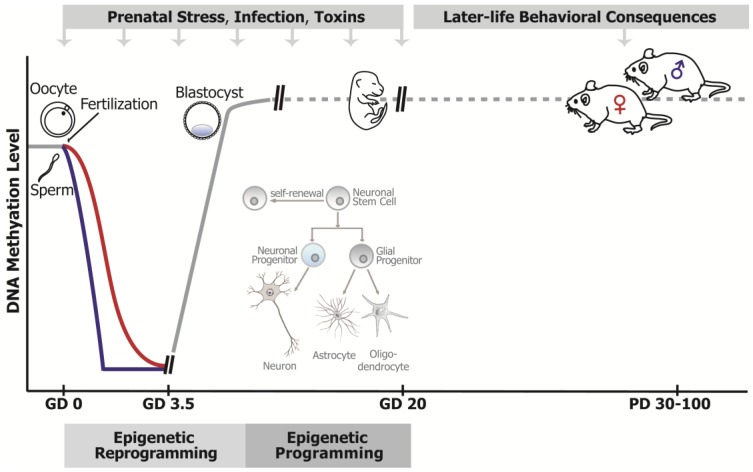
Figure 1.
The epigenome as a substrate for the lasting effects of prenatal stressors on brain function and behavior. The epigenome is particularly vulnerable to disruption by environmental agents during prenatal development, when an extensive reprogramming and programming of epigenetic modifications takes place. The post-fertilization “epigenetic reprogramming” (zygote to blastocyte stage) includes the almost complete erasure of DNA methylation in both the paternal (blue line) and the maternal (red line) genome, which is then re-established (solid gray line), leading to differential DNA methylation and gene expression patterns in the first cell lineages. In the later stages of development, epigenetic marks are less dynamic (dashed gray line), but still actively participate in gene expression programming, relevant for later stages of cellular differentiation (“epigenetic programming”). As an example, during the differentiation of brain cells (see picture inset), DNA methylation and histone modifications are involved in the gene expression programming that differentiates neuronal stem cells into neuronal and glial progenitors, and further into more specialized neuronal and glial cells (astrocytes and oligodendrocytes). Hence, prenatal exposure to environmental factors that affect the epigenome (stress, infection, toxins) can disrupt gene expression programming in the embryo/fetus, resulting in developmental deficits, including abnormal brain development that can lead to later-life behavioral disorders. Importantly, the epigenome is also dynamic in mature, postmitotic neurons (depicted as a dashed gray line postnatally), so long-term behavioral abnormalities may also result from the improper developmental programming of the brain’s epigenetic machinery that continues to be used by mature neurons.