Abstract
Objective
In addition to adipocytes, adipose tissue contains large numbers of immune cells. A wide range of evidence links the activity of these cells to regulation of adipocyte and systemic metabolic function. Bariatric surgery improves several aspects of metabolic derangements and at least some of these effects occur in a weight-loss independent manner. We sought to investigate the impact of vertical sleeve gastrectomy (VSG) on adipose immune cell frequencies.
Methods
We analyzed the frequencies of immune cells within distinct adipose tissue depots in obese mice that had VSG or sham surgery with a portion of the latter group pair-fed such that their body mass was matched to the VSG animals.
Results
We demonstrate that VSG induced a shift in the epididymal adipose tissue leukocyte profile including increased frequencies of CD11c− macrophages, increased frequencies of T cells (CD4+, CD8+, and CD4−/CD8− T cells all increased), but a significantly decreased frequency of adipose tissue dendritic cells (ATDC) that, despite the continued high fat feeding of the VSG group, dropped below control diet levels.
Conclusions
These results indicate that VSG induces substantial changes in the immune populations residing in the adipose depots independent of weight loss.
Keywords: Sleeve gastrectomy, Adipose tissue, Macrophages, T cells, Dendritic cells
Abbreviations: ATDC, adipose tissue dendritic cell, ATM, adipose tissue macrophage, eWAT, epididymal white adipose tissue, FFA, free fatty acids, HFS, high fat sham, iWAT, inguinal white adipose tissue, SVC, stromal vascular cells, VSG, vertical sleeve gastrectomy
Highlights
-
•
Sleeve gastrectomy in mice altered the adipose leukocyte populations.
-
•
CD11c− macrophages were selectively upregulated independent from weight loss.
-
•
All identified T cell populations increased manifold independent from weight loss.
-
•
The dendritic cell fraction was decreased below lean control levels with surgery.
Fact box on the identified immune cell populations
CDC11c− ATMs
The resident macrophage population found in lean adipose tissue expresses relatively higher expression of genes typically up-regulated in alternatively activated M2-like macrophages including CD206 and Arginase. These macrophages express little to no pro-inflammatory cytokines and are thought to potentially play a role in maintaining adipose tissue homeostasis.
CD11c+ ATMs
CD11c+ macrophages accumulate in adipose tissue during obesity where they cluster around dying adipocytes forming what’s known as crown-like structures. They have increased expression of pro-inflammatory genes and have been associated with glucose intolerance and insulin resistance.
CD4+ T cells
CD4 is a co-receptor expressed on a subset of T cells that respond to antigen presented by antigen presenting cells expressing MHC-II. CD4+ T cells can be further subdivided between helper T cells, which provide cytokine signaling help to other leukocytes, and regulatory Foxp3+ T cells which modulate and control inflammatory responses. During obesity pro-inflammatory TH1 cells, which express IFNγ, accumulate in adipose tissue and eventually outnumber the regulatory T cells and TH2 cells present in lean adipose tissue.
CD8+ T cells
T cells expressing CD8 can be activated to become cytotoxic T cells which produce high amounts of IFNγ and are capable of killing other cells in an antigen-specific manner. Activated CD8+ T cells accumulate in visceral adipose tissue with obesity and are thought to contribute to the inflammatory milieu.
ATDC
ATDCs are one of the professional antigen presenting cells, along with macrophages and B cells, meaning they can present antigen to, and activate, CD4+ or CD8+ T cells. Their role in adipose tissue is not well understood and has been complicated by limited unique surface markers that would allow for separation of ATMs from ATDCs.
1. Introduction
Bariatric surgery is currently the most successful treatment for obesity and is the only therapeutic option that also causes sustained substantial reduction of type 2 diabetes [1]. Consequently, there is great interest in understanding the underlying mechanisms that produce these potent benefits. While many have hypothesized “mechanical” explanations for these effects (i.e. meal-size restriction and/or malabsorption), such mechanisms do not account for the broad effects on physiology seen after surgery [2], [3]. In support of this, the metabolic effects of the two most commonly used operations, Roux-en Y gastric bypass and vertical sleeve gastrectomy (VSG), result in surprisingly similar physiologic and behavioral effects despite the gross anatomic differences. Both surgeries alter gut hormones, bile acids, insulin sensitivity, and inflammatory markers in a manner that is independent of weight loss and is hypothesized to have significant impact on systemic metabolism [4]. Adipose tissue itself also shows weight-independent changes in response to bariatric surgery, such as alterations in fat pad distribution and adipocyte size, adipokine secretion, and inflammatory markers [5], [6], [7], [8], which underscores a potential link between adipose tissue and systemic metabolic improvements after surgery.
The hallmarks of obesity include adipocyte hypertrophy, fat mass expansion, and increased accumulation within the adipose tissue of leukocytes involved in both innate and adaptive immune responses. Obese visceral fat contains more than 1 million immune cells per gram of tissue [9]. These leukocytes contribute to local, chronic inflammation and have been hypothesized to drive obesity-associated insulin resistance as several inflammatory cytokines and adipokines interfere directly with insulin sensitivity and insulin secretion [10], [11]. Macrophages make up a majority of the leukocyte infiltrate in obese adipose tissue. These adipose tissue macrophages (ATMs) tend to polarize towards a pro-inflammatory phenotype [12]. Two primary types of ATMs have been identified based on the expression of CD11c. Lean mice and humans have a preponderance of a resident population of CDllc− ATMs while individuals with obesity have an accumulation of CD11c+ ATMs that have a lysosomal activation phenotype [13]. Adipose tissue dendritic cells (ATDCs) are another distinct myeloid cell population that independently contributes to adipose tissue inflammation [14]. Both CD8+ and CD4+ T cells also accumulate in adipose tissue of obese animals and are thought to partner with ATMs to promote a pro-inflammatory environment [15], [16]. Recent studies suggest that not only do leukocytes impact the function of the adipocytes but adipocytes can also alter immune cell responses – both by mechanisms that involve non-inflammatory interactions [17]. In addition to this, it is well established that the gut microbiota changes with bariatric surgery as well [18], [19], and these changes may alter overall immune function as well.
In light of this, the goal of our study was to investigate if VSG would change adipose tissue leukocyte populations differently from identical weight loss obtained by calorie restriction in the context of obesity.
2. Research designs and methods
2.1. Animals and surgery
All animal experiments were approved by the Institutional Animal Care & Use Committee at the University of Michigan (Animal Use Protocol # PRO00005678). Six weeks old male C57BL6/J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were initially group housed and fed a 60% high-fat diet (D12492, Research Diets, New Brunswick, NJ, USA) or control diet (5LOD irradiated diet, Labdiet, St. Louis, MO, USA), ad libitum for 12 weeks prior to surgery. Two weeks prior to surgery, the mice were single-housed. Mice were then stratified into 4 different groups based on bodyweight immediately before surgery: 1) Lean control group (control), mice maintained on control diet throughout the experiment with no surgery performed; 2) High fat sham group (HFS), mice maintained on ad libitum high fat diet throughout the study and receiving sham surgery to control for effect of surgery; 3) VSG group (VSG), mice maintained on ad libitum high fat diet throughout the study both before and after receiving VSG surgery. Food intake was monitored on a daily basis after surgery. 4) Pair-fed group (pair-fed), mice with ad libitum access to high fat diet before sham surgery. After sham surgery, pair-fed mice were administered the average amount of high fat diet ingested by the VSG group the day before. VSG and sham surgeries were performed under isoflurane anesthesia as described previously [20]. Briefly, the lateral 80% of the stomach was resected, leaving a tubular gastric remnant in continuity with the esophagus proximally and the pylorus distally. The sham procedure involved the application of pressure on the stomach with blunt forceps along a vertical line between the esophageal sphincter and the pylorus. The first 4 days following surgery, the mice were administered a liquid diet (Osmolite 1 Cal). A few postoperative deaths (mainly within the first few days after surgery) yielded final group numbers of n = 12 for HFS mice, n = 12 in the pair-fed group, and n = 13 in the VSG group. Body weights were measured daily for the first 2 weeks after surgery and then weekly thereafter. Body composition analysis took place 8 weeks after surgery (EchoMRI, Echo Medical Systems, Houston, TX, USA)
2.2. Blood sampling and analysis
Fed and fasted plasma levels of non-esterified free fatty acids (FFA) were sampled 5 weeks after surgery. Fed plasma FFA samples were obtained 1 h after the dark cycle to match feeding initiation. Blood was obtained by cutting the tail and collecting blood in tubes containing NaF (Ram Scientific, Yonkers, NY, USA). Immediately after this initial blood sampling, mice were fasted for 16 h before another blood sampling was performed. Plasma FFA was analyzed with a fluorometric FFA assay kit (Cell Biolabs, San Diego, CA, USA), and delta FFA was calculated as FFA(fasted) minus FFA(fed). Blood sampling for insulin took place 6 weeks after surgery. Food was removed in the morning and blood was obtained via the tail vein after a 4 h fast. Blood glucose measurements were performed with handheld glucometers. Insulin levels were measured using an ultrasensitive mouse insulin ELISA kit (Chrystal Chem, Downers Grove, IL, USA).
2.3. Euthanasia and fat pad processing for immune cell analysis
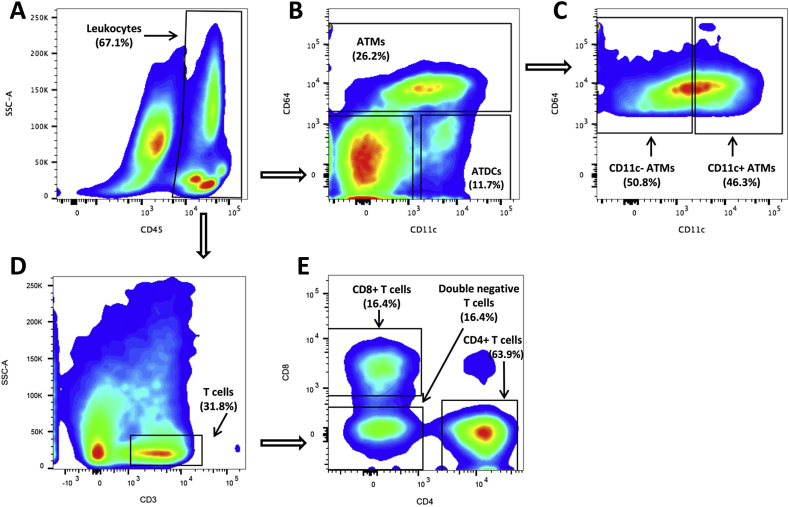
On the day of euthanasia, mice were anesthetized with isoflurane and blood was collected into EDTA vacutainers after a cardiac puncture. Immediately after this, bilateral pneumothorax was induced, and the carcass was perfused with 10 ml PBS before fat pads were excised. Epididymal (eWAT) and inguinal (iWAT) white adipose tissues were dissected whole and weighed. Tissues were stored in PBS until mincing in RPMI culture medium (Gibco, Gaithersberg, MD, USA) containing 0.5% BSA. Collagenase (Type II; Sigma–Aldrich Inc., St Louis, MO, USA) was added to a final concentration of 1 mg/ml and tissue suspensions were incubated at 37 °C for 40 min with constant shaking. The resulting cell suspensions were filtered through a 100-μm filter and centrifuged at 500g for 10 min to separate floating adipocytes from the stromal vascular cells (SVC)-containing pellet. For flow cytometry, SVCs were incubated in 0.5 ml RBC lysis buffer for 5 min at room temperature and then resuspended in PBS/0.5% BSA prior to incubation in Fc Block (rat anti-mouse CD16/CD32; eBioscience, San Diego, CA, USA) for 15 min on ice. Cells were stained with the indicated antibodies for 30 min at 4 °C in the dark (CD45, CD8, CD3, CD4, and CD11c, eBioscience, San Diego, CA. CD64, BD Pharmingen, Franklin Lakes, NJ, USA). Stained cells were washed twice in PBS and fixed in 0.1% paraformaldehyde. Cells were analyzed using a FACSCanto II Flow Cytometer (BD Biosciences) and FlowJo flow cytometry software (Treestar Inc., Ashland, OR, USA). Scatter plots showing the strategy for identifying the different immune populations is given in Figure 2.
Figure 2.
Scatter plots for identified immune populations. Scatter plots including gating strategy for all identified immune cell sub populations within a representative eWAT sample from the HFS group. A) Leukocytes were identified as CD45+ positive cells of the stromal vascular fraction of cells present in adipose tissue. B) ATMs were gated off the CD45+ leukocytes and identified as being CD64+. Dendritic cells were gated off the CD45+ leukocytes and identified as being CD64−/CD11c+. C) ATMs were further identified as being CD11c− or CD11c+. D) T cells were gated off the CD45+ leukocyte population and identified as CD3+. E) T-cells subpopulation were gated off the CD3+ T-cell population and identified as CD8+, CD4+, or double negative (CD4−/CD8−).
2.4. Plasma cytokines
Plasma obtained by cardiac puncture at the time of euthanasia was analyzed by V-PLEX Plus Proinflammatory Panel 1 (mouse) Kit (Meso Scale Diagnostics, Rockville, MD, USA) according to the manufacturers guidelines.
2.5. Statistics
Statistical significance was tested by one-way ANOVA followed by Tukey pairwise comparisons. To reduce the statistical symbols included in the data figures (Figure 3, Figure 4), the post hoc tests were reduced to show only the effect of high fat diet feeding (control versus HFS), weight loss (HFS versus pair-fed), and weight independent effect of surgery (pair-fed versus VSG). For an extensive overview of the statistical differences between groups, tables of p values are given for all groups compared to all groups in Supplementary Table 1. Prior to statistical analysis, the ROUT outlier test with Q = 1% was applied to all datasets to exclude potential outliers. Datasets that did not meet the requirements for equal variances were logarithm or square root transformed prior to analysis. p < 0.05 was considered significant for all tests. Statistical analyses were performed in Prism (version 5, GraphPad Software, Inc., La Jolla, CA, USA).
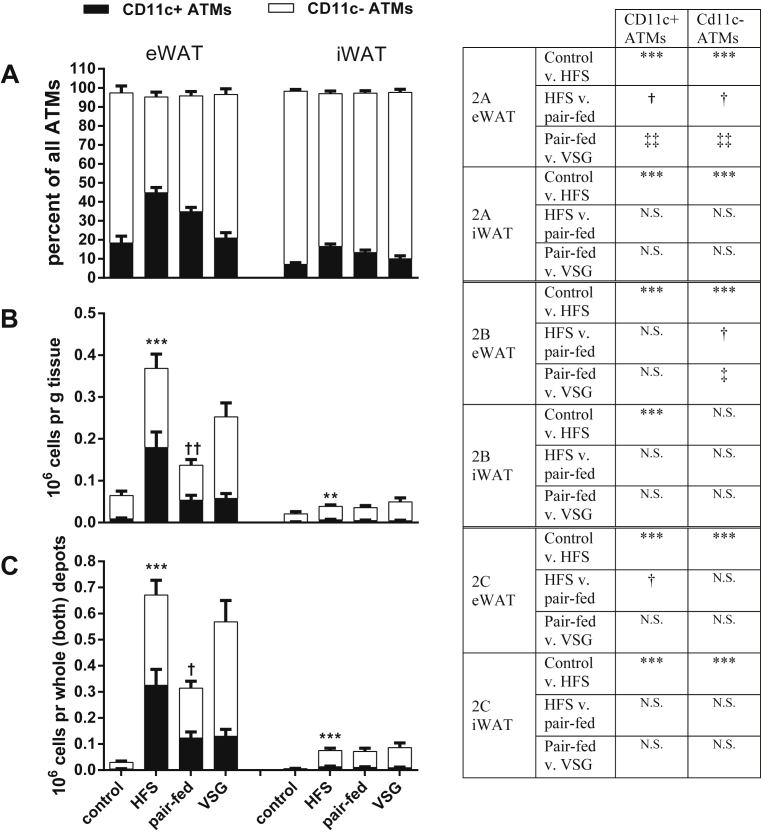
Figure 3.
ATMs. CD11c+ and CD11c− ATMs in eWAT and iWAT, respectively. A) Percentage of all ATMs that are CD11c+ and CD11c−, respectively. B) Number of ATMs per g tissue that are CD11c+ and CD11c−, respectively. C) Number of ATMs in whole depot that are CD11c+ and CD11c−, respectively. Symbols over the bars represent significant differences between the total ATM populations. Significant differences between CD11c+ ATMs or CD11c− ATMs, respectively, are given in the table to the right of the graphs. ***p < 0.001 for control group versus HFS (effect of high fat diet); †p < 0.05, ††p < 0.01 for HFS compared to pair-fed group (effect of weight loss); ‡‡p < 0.01 for pair-fed as compared to VSG group (weight independent effect of VSG). Tukey post-hoc tests following a one-way ANOVA. Data are mean ± SEM. N.S. is non significance between groups.
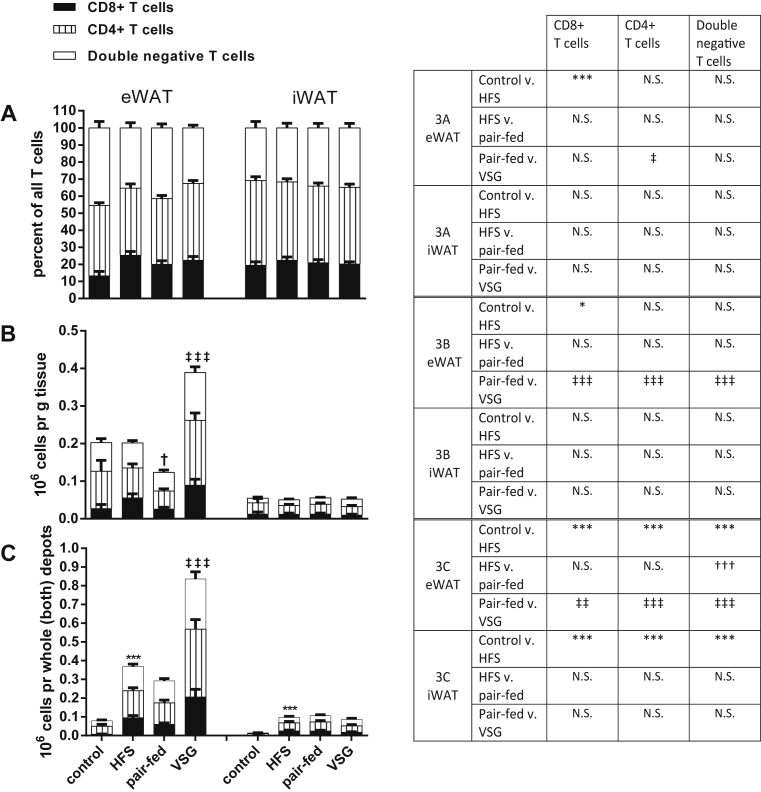
Figure 4.
T cells. T cell populations within eWAT and iWAT, respectively. T cells are either CD3+ T cells, CD8+ T cells or double negative T cells. Statistical significance of differences between groups are given by symbols on top of the bars for the total height of the bar or by symbols in the table to the right for the individual T cell populations. A) Stacked bars representing the percentage contribution of each T cell type within the entire T cell population. B) Stacked bars representing the individual T cell populations as millions per g tissue. C) Stacked bars representing the individual T cell populations as millions per whole depot. *p < 0.05, **p < 0.01, ***p < 0.001 for control diet versus HFS group (effect of high fat diet); †p < 0.05, ††p < 0.01, †††p < 0.001 for HFS compared to the pair-fed group (effect of weight loss); ‡p < 0.05, ‡‡p < 0.01, ‡‡‡p < 0.001 for pair-fed as compared to VSG group (weight independent effect of VSG). Tukey post-hoc tests upon one-way ANOVA. Error bars are SEM. N.S. is non significance between groups.
3. Results
3.1. Bodyweight and metabolic characteristics
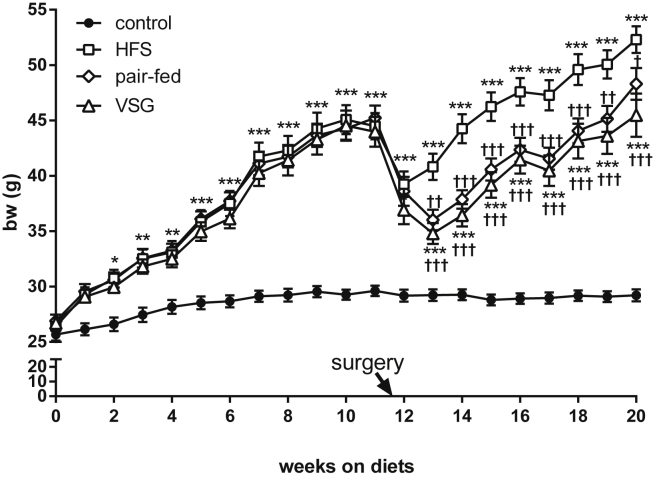
Twelve weeks into the study control diet fed mice weighed 29.6 ± 1.5 g and all high fat diet fed mice weighed 44.6 ± 4.3 g (Figure 1). By two weeks after surgery and throughout the remainder of the study, the VSG group weighed significantly less than the HFS group. By design, the pair-fed group had a similar body mass trajectory as the VSG mice. Food intake in the HFS group was significantly greater than in the VSG group for the first two weeks (data not shown) but did not differ thereafter (data not shown).
Figure 1.
Body weight. Body weight trajectory throughout the study. Control mice maintained on control diet throughout the experiment (black circles), HFS group (white squares), pair-fed mice (white rhombus), and VSG group (white triangles). All mice had ad libitum access to food throughout the study except the HF pair-fed group. After surgery, these mice were fed the amount of food ingested by the VSG group. Arrow at the x-axis indicates surgery day. *p < 0.05, **p < 0.01, ***p < 0.001 as compared to chow control group; †p < 0.05, ††p < 0.01, †††p < 0.001 as compared to HF sham (Tukey post-hoc tests upon one-way ANOVA). Data are mean ± SEM.
At the time of euthanasia all 3 groups maintained on high fat diet had significantly higher bodyweight than the control group (Table 1). Lean body mass was slightly elevated in all high fat diet fed groups, but there was no impact of surgery on lean mass (Table 1). The HFS group gained more weight via body fat compared to the VSG group. There were no significant differences between the VSG and the pair-fed group for any of these parameters. iWAT and eWAT weights were higher in all high fat diet fed groups as compared to the control diet group, but there was no significant reduction in adipose tissue weight with VSG.
Table 1.
Metabolic parameters.
| Measure | Time post-surgery | Control | HFS | Pair-fed | VSG |
|---|---|---|---|---|---|
| Total body weight (g) | 8 weeks | 29.2 ± 1.7 | 52.3 ± 4.2*** | 48.3 ± 4.9*** | 45.5 ± 7.0***;†† |
| Weight gain after surgery (g) | 8 weeks | −0.4 ± 1.0 | 7.8 ± 2.5** | 3.1 ± 2.9 | 1.5 ± 5.4†† |
| Fat mass (g) | 8 weeks | 2.7 ± 0.4 | 19.5 ± 2.9*** | 16.1 ± 3.1*** | 14.9 ± 5.0***;†† |
| Lean body mass (g) | 8 weeks | 23.6 ± 1.1 | 26.5 ± 1.4*** | 25.3 ± 1.5* | 25.8 ± 1.4** |
| eWAT weight (g) | 8 weeks | 0.45 ± 0.12 | 2.03 ± 0.64*** | 2.36 ± 0.46*** | 2.28 ± 0.91*** |
| iWAT weight (g) | 8 weeks | 0.26 ± 0.05 | 1.88 ± 0.31*** | 1.86 ± 0.45*** | 1.73 ± 0.82*** |
| Plasma insulin after 4 h fast (ng/ml) | 6 weeks | 0.38 ± 0.09 | 2.75 ± 1.75*** | 1.79 ± 0.73*** | 2.01 ± 1.16*** |
| Blood glucose after 4 h fast (mg/dl) | 6 weeks | 135 ± 18 | 190 ± 18*** | 164 ± 26 | 178 ± 35** |
| Plasma FFA in the fed state (uM) | 5 weeks | 173 ± 57 | 244 ± 63 | 243 ± 63 | 213 ± 95 |
| Plasma FFA after 16 h fast (uM) | 5 weeks | 435 ± 75 | 408 ± 94 | 356 ± 93 | 432 ± 138 |
| Delta FFA (fast minus fed) (uM) | 5 weeks | 262 ± 62 | 164 ± 106 | 113 ± 61** | 219 ± 118‡ |
End points of metabolic relevance. Values are given as mean ± std deviation. *p < 0.05, **p < 0.01, ***p < 0.001 as compared to the chow group; †p < 0.05, ††p < 0.01, ††p < 0.001 as compared to the HFD group; ‡‡p < 0.01, ‡‡‡p < 0.001 as compared to the pair-fed group.
With high fat diet feeding, as compared to control diet feeding, glucose and insulin levels were compromised, reflected in both being significantly elevated after a 4 h fast. However, no significant improvement was found with VSG in this study. Plasma FFA in the fed and the fasted state, respectively, did not differ between any of the groups. Yet, when calculating the delta FFA values representing the capacity of adipose tissue to adapt to change in energy status with fasting, there was a significant weight-loss independent improvement with VSG
3.2. ATM populations
Within the adipose tissues, CD45+/CD64+ cells (see Figure 2) were identified as ATMs and then further characterized as CD11c+ or CD11c− (for a description of these ATM subtypes see the Fact Box). In both eWAT and iWAT, the majority of macrophages were CD11c− (Figure 3A). The frequency of CD11c+ macrophages increased with high fat diet feeding in both eWAT and iWAT. Weight loss (pair-fed compared to HFS) led to significant reduction of the frequency of CD11c+ ATMs in eWAT but not iWAT, and VSG caused a weight-independent reduction of CD11c+ ATMs (VSG compared to pair-fed).
Expressing the ATM content relative to tissue weight (Figure 3B) demonstrated that high fat diet feeding greatly increased the number of CD11c+ ATMs in eWAT and iWAT. With weight loss there was a large reduction in the overall number of ATMs (pair-fed versus HFS). Despite the lack of significant changes in weight-independent effect of VSG on total ATM number, Figure 3B indicates that the relative increase in CD11c− ATMs was due to more of these ATMs being present rather than a reduction in the CD11c+ type.
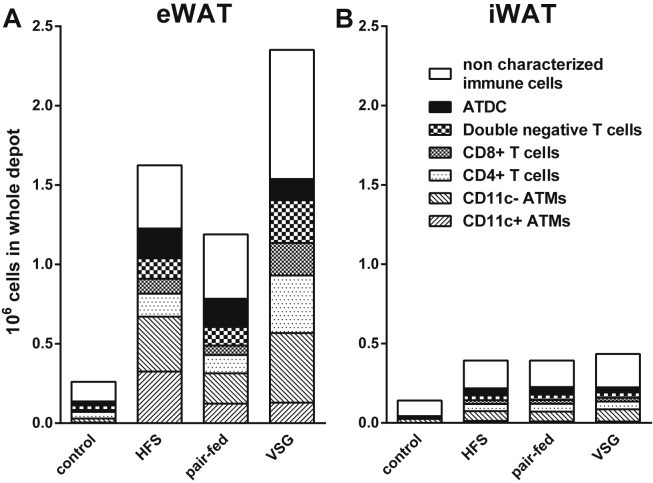
Expressing ATMs relative to the whole depot (Figure 3C) revealed a similar pattern as when expressed relative to tissue weight. The number of CD11c+ ATMs was more than 70-fold higher in the HFS group compared to the controls and 13-fold higher for the CD11c− ATMs, which gives a powerful indication of how many macrophages are recruited with obesity. When depicting the total number of all macrophages together with all other immune cells (Figure 6), it was found that there were fewer macrophages per total number of immune cells in the eWAT with surgery (40.21 ± 11.3% in HFS versus 22.27 ± 2.98% in the VSG group. p < 0.001, one-way ANOVA with Tukey post hoc test).
Figure 6.
Total leukocyte numbers within eWAT and iWAT. Total number of leukocytes identified as being either ATMs, T-cells or ATDCs in eWAT (A) and iWAT (B), respectively.
3.3. T cell populations
In the current study, we identified T cells as being CD45+/CD3+. This population was further divided into CD4+ T cells and CD8+ T cells as well as the double negative T cells (CD4−/CD8−) (see Figure 2 as well as the Fact Box for a description of each of the T cell types). Figure 4A shows the relative frequencies of the subpopulations of all CD3+ T cells. In eWAT, the CD8+ T cell population was increased with high fat diet feeding in general, but there was no effect of VSG. In contrast, the frequency of CD4+ T cells was increased with VSG compared to HFS and pair-fed groups (see statistics in Supplementary Table 1). The double negative T cells did not respond to diet but were decreased in the VSG group as compared to controls (see statistics in Supplementary Table 1). No changes in T cell frequency were observed within the iWAT.
When calculating the number of T cells relative to tissue weight (Figure 4B), there was a significant weight independent upregulation of the number of T cells per g eWAT with VSG within all subpopulations. The T cell response in iWAT, however, did not change with diet or surgery. The T cell numbers relative to the whole depot (Figure 4C) showed that manifold T cells were present with high fat diet feeding in both eWAT and iWAT.
Within the high fat diet fed groups, regardless of surgery, the pattern remained similar to what was observed by expressing the cell content relative to tissue weight. Figure 6A shows that the increase in T cells in eWAT is not matched by any of the other types of immune cells, leaving T cells to constitute a larger proportion of the leukocyte population than any of the other groups.
3.4. ATDC cell population
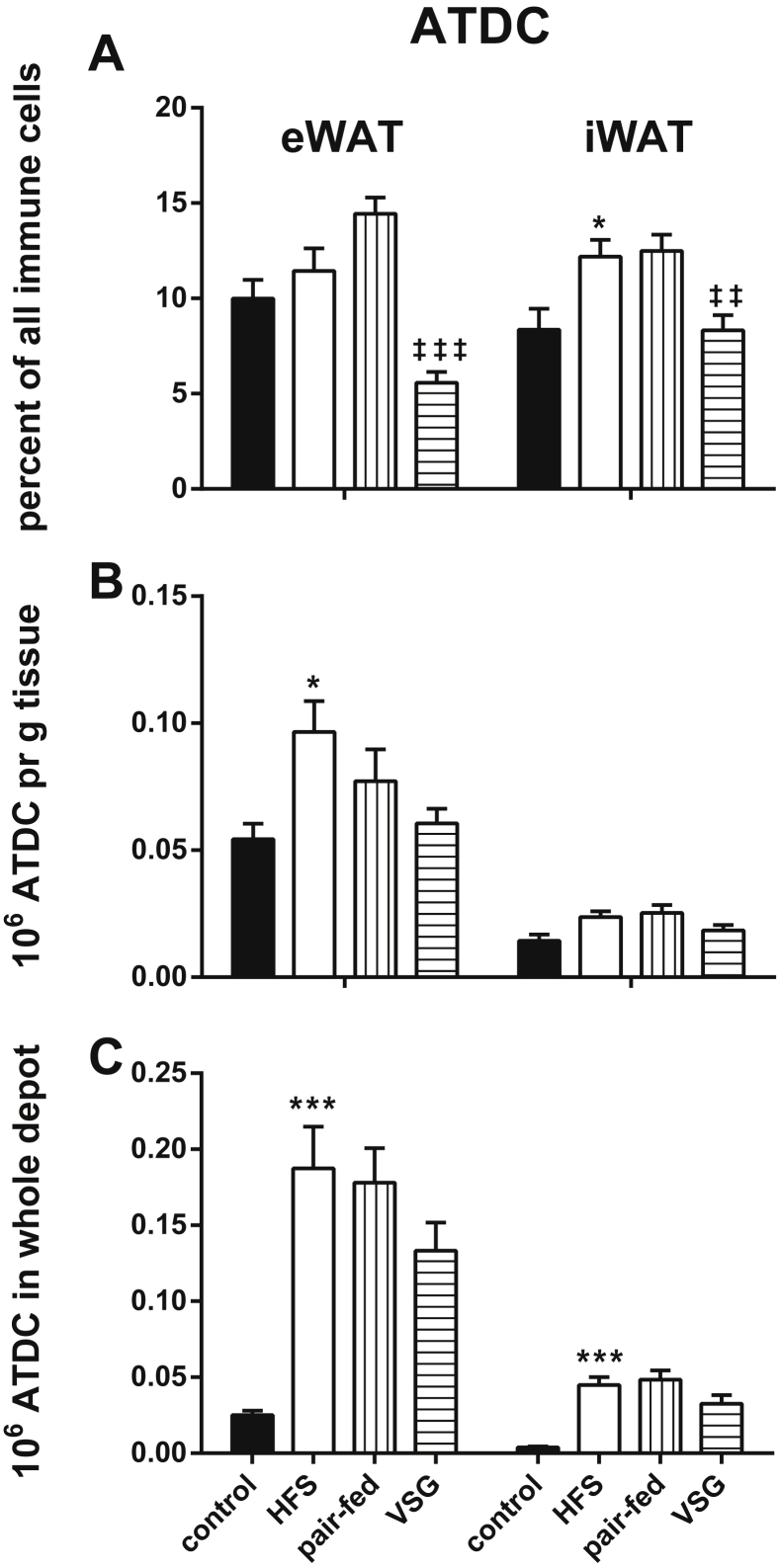
ATDCs were identified as CD45+/CD64−/CD11c+ positive cells (see Figure 2 as well as the Fact Box for a description of this cell type). As there was no further characterization of dendritic cell subpopulations in the current study, their frequency distribution was characterized as percentage of all immune cells (Figure 5A). Overall, we found that even though the total number of ATDCs increased with high fat feeding in both depots (Figure 5B,C), this was not reflected with a similar magnitude of change in the ratio of ATDCs to all leukocytes. With VSG, however, the number of ATDCs did not change (Figure 5B,C) whereas the ratio of ATDCs to all other immune cells significantly decreased to levels that were lower than in the control group in both eWAT and iWAT (Figure 5A).
Figure 5.
ATDCs. ATDC frequencies within eWAT and iWAT, respectively. A) Percent of entire immune cell population consisting of ATDC. B) Number of ATDCs given as millions per g tissue. C) Number of ATDCs given as millions per whole depot. *p < 0.05, ***p < 0.001 for control diet fed versus HFS group (effect of high fat diet); ‡‡p < 0.01, ‡‡‡p < 0.001 for pair-fed as compared to VSG group (weight independent effect of VSG). Tukey post-hoc tests upon one-way ANOVA. Error bars are SEM. N.S. is non significance between groups.
3.5. Plasma cytokines
Plasma cytokines (IL-1β, IL-2, IL-5, IL-6, IL-10, IFNγ, and TNFɑ) were measured in samples obtained at the time of euthanasia, and the values are given in Table 2. High fat diet feeding resulted in significant increases of IL-2 and IFNγ. No changes were found between the HFS and pair-fed groups. Yet with VSG all measured cytokines, except IL-2 and IL-5, increased manifold as compared to the weight matched pair-fed group.
Table 2.
Plasma cytokines.
| Cytokine | Control | HFS | Pair-fed | VSG |
|---|---|---|---|---|
| IL-1β (pg/ml) | 0.566 ± 0.630 | 0.236 ± 0.010 | 0.142 ± 0.076* | 1.73 ± 1.33*,†††,‡‡‡ |
| IL-2 (pg/ml) | 2.65 ± 2.16 | 0.833 ± 0.412* | 0.945 ± 0.193 | 1.85 ± 0.698†† |
| IL-5 (pg/ml) | 2.98 ± 1.16 | 2.70 ± 0.632 | 2.08 ± 0.612 | 1.83 ± 0.545* |
| IL-6 (pg/ml) | 8.62 ± 5.26 | 16.5 ± 9.80 | 10.5 ± 5.24 | 79.5 ± 70.7***,†††,‡‡‡ |
| IL-10 (pg/ml) | 16.8 ± 3.89 | 20.4 ± 5.47 | 14.1 ± 2.89 | 37.0 ± 15.7***,††,‡‡‡ |
| IFNγ (pg/ml) | 1.84 ± 1.54 | 0.241 ± 0.0681** | 0.302 ± 0.128* | 1.82 ± 1.52†††,‡‡ |
| TNFɑ (pg/ml) | 9.10 ± 3.48 | 10.5 ± 5.04 | 7.60 ± 2.59 | 20.9 ± 10.9**,†,‡‡‡ |
Plasma cytokine concentrations. Values are given as mean ± std deviation. *p < 0.05, **p < 0.01, ***p < 0.001 as compared to control diet group; †p < 0.05, ††p < 0.01, †††p < 0.001 as compared to HFS group; ‡‡p < 0.01, ‡‡‡p < 0.001 as compared to pair-fed group.
4. Discussion
4.1. VSG's effect on ATMs
In this study, we found several weight-loss independent effects of VSG on the adipose tissue immune populations. As expected, high fat feeding caused an accumulation of CD11c+ ATMs that was reversed with weight loss (Figure 3B,C). But with VSG, more CD11c− ATMs were found in the tissue than in the pair-fed group (Figure 3B,C). The end result being that the ratio between these two types of ATMs was brought back to control levels (Figure 3A). The CD11c− ATMs are known to be involved in adipose tissue remodeling [21], and these data indicate that VSG might improve this function helping the adipose tissue to remodel as weight is rapidly lost. An additional issue raised by these results is the potential for caloric restriction to increase lipolysis, which can alter leukocyte populations within the adipose tissues [22]. However, in the current study, food intake in the VSG and pair-fed groups was only decreased during the initial weeks after surgery, which is a common effect found after VSG in mouse models [23]. For the remaining 6 weeks of this study, there was no significant difference in food intake as compared to the ad libitum fed HFS mice, which limits the degree to which this may have contributed to the current results.
4.2. VSG's effect on T cells
With obesity, an accumulation of CD8+ and CD4+ T cells precedes macrophage infiltration and may promote their recruitment to obese adipose tissue [15]. In our study, we found that high fat diet feeding induced an increased frequency of CD8+ cells whereas with VSG there was a weight-independent relative increase in the frequency of CD4+ T cells out of all T-cells (Figure 4A). This change brought the ratio between these two populations of T cells back towards the lean phenotype. Yet the most drastic change in the T cell populations was the manifold increase in numbers (Figure 4B,C). From this dataset, we cannot conclude on the further characterization of these T cell populations in terms of pro- or anti-inflammatory phenotypes nor on the source of this T cell accumulation. There are reports, however, that T cell proliferation is altered in mice exposed to gut microbiota from high fat fed mice [24]. Given that the microbiome is profoundly altered after bariatric surgery and has been linked to other beneficial effects of surgery [25], it opens up the hypothesis that alterations in the microbiome contribute to alterations in T cell populations that occur after VSG.
4.3. VSG's effect on ATDCs
We observed that the fraction of ATDC of all immune cells was drastically reduced with surgery in eWAT as well as iWAT (Figure 4A). This could be an artifact of other cell types being increased with surgery (such as the T cells). Nevertheless, it is possible that the reduced ratio of ATDCs can contribute to altered immune function in adipose tissue that may contribute to improvements in metabolic function. Very little is known about the function of ATDCs in adipose tissues. A report from Zlotnikov-Klionzky et al. unexpectedly found that deletion of perforin positive dendritic cell populations had a high impact on metabolic phenotype as mice lacking perforin positive dendritic cells gained weight while being maintained on a control diet, were glucose intolerant and insulin resistant, and had increased blood lipids [26]. ATDCs may also contribute positively towards promoting adipose tissue inflammation and are regulated by CCR7-dependent recruitment into adipose tissue of obese mice [14]. Our observations suggest that signals that sustain ATDCs may be dampened by VSG. Since both ATM and ATDC have the capacity to control T cell fates as antigen presenting cells, it is possible that the suppression of ATDC and the expansion of adipose tissue T cells are mechanistically linked.
4.4. Possible mechanisms for how VSG can alter the leukocyte populations
A key question emerges with this data set. How can VSG produce these alterations in the immune populations in adipose tissue? The current data cannot answer this question, but it is well documented that bariatric surgeries produce dramatic and long-lasting alterations to the gut microbiota [18], [19], and T cells from the gut can migrate to adipose tissue [27]. These findings raise the possibility that microbiota changes could manifest themselves as changes in immune population in other sites. Another possible link between surgery and the immune system is changing bile acid levels. Both VSG and RYGB are associated with increased circulating bile acids [28]. Bile acids have been shown to reduce the pro-inflammatory capability of macrophages [29], and we have previously reported that mice that lacked the nuclear bile acid receptor FXR had dramatically reduced effects of VSG on both body weight and glucose regulation [23].
4.5. Metabolic parameters and cytokine plasma levels
As stated in the introduction, bariatric surgery not only causes long lasting weight loss with the associated health benefits but also improves glucose control in a weight independent manner. Mice exposed to the high fat diet gained substantial body fat when compared to the control group (Figure 1), and insulin and glucose levels were both elevated. We did not find weight independent improvements in glucose and insulin levels after 4 h of fasting with VSG, yet these changes are often more difficult to detect in mouse models after surgery [30], [31], [32]. The capacity of the adipose tissue to release FFA as a response to fasting (Table 1) was not significantly undermined by high fat feeding albeit there was a trend for increased levels in the fasted state. In contrast, we found that the ability of the adipose tissue to increase FFA secretion when transitioning from the fed to the fasted state (delta FFA) was improved with surgery, which suggests an improved metabolic responsiveness of the adipose tissue per se.
The cytokine levels in plasma detected with high fat feeding were remarkably similar to the control group with only IL-2 levels decreasing and IFNγ levels increasing (Table 2). Despite obesity being categorized as a low chronic inflammatory condition, the cytokine changes in plasma from high fat fed mice as compared to lean control mice are often small in magnitude [33], [34], [35]. Yet in the VSG group, all measured cytokines changed drastically. Several cytokines were upregulated (IL-1β, IL-6, IFN-γ, and IL-10), and these were a mix of pro-inflammatory (IL-1, IFN-γ) and anti-inflammatory (IL-10, IL-6) cytokines. Whether this is relevant to the metabolic improvements observed after surgery is unclear, yet it indicates that the immune environment is significantly altered with VSG which fits with the potent upregulation of T cells present in the eWAT. Recently, there has also been evidence that some cytokines can act directly on systemic metabolic changes via immune cells, such as IL-1β being produced by peritoneal macrophages in response to feeding and subsequently acting on the pancreatic β cells to increase insulin secretion [36]. In the light of this, there is even more reason to believe that changes in the immune cells/cytokines after surgery could be relevant to metabolic improvements after surgery.
Overall, these findings suggest that VSG drastically altered the systemic inflammatory environment without causing either disturbances or improvements in glucose metabolism. In contrast, the adipose tissue responsiveness to feeding and fasting was improved. Despite the majority of changes being found in the eWAT only, this could still be considered of relevance to the metabolic response as the visceral adipose tissue is considered far more metabolically active and as it contains far greater number of leukocytes [37]. In addition to the described changes in the ATMs, the T cells, and the ATDCs, Figure 6 also shows that there are non-characterized leukocyte populations that increase in number with diet (eWAT and iWAT) and surgery (only eWAT). This population most likely represents neutrophils, mast cells, and eosinophils. Future work will be required to identify these cell types.
4.6. Potential metabolic impact of the leukocyte changes
In our study, we found that many of the weight-independent effects of VSG in adipose tissue resulted in immune cell populations changes that were more similar to the patterns found in the control group than in the HFS group. Interestingly, several of these changes took place in a manner that could contribute towards metabolic improvement. These included fewer CD11c+ ATMs and a 3-fold increase in CD4+ T cells and double-negative T cells. The CD11c+ ATMs are commonly upregulated with adipose inflammation, and CD11c− ATMs have a role in remodeling of adipose tissue [21]. Overall, this suggests that the adipose tissue has a greater capacity to recover from obese inflammation after VSG than after calorie restriction. The CD4+ T regulatory cells have been shown to be responsible for maintaining insulin sensitivity, and boosting the number of these cells can improve the insulin sensitivity in obese mice [38]. In this study, we did not see significant improvement of insulin sensitivity with VSG, an effect that can be hard to detect in mouse models [30], [31], [32]. Hence, we cannot confirm nor reject the metabolic significance of these T cell changes. It is important to keep in mind, however, that we only investigated the T cells in the adipose tissue and that we did indeed find an improved metabolic responsiveness of this tissue to secrete FFAs. T cell populations in other organs or the circulation could have shown other changes. To the best of our knowledge, there are no reports on the double-negative T cells being linked to metabolic outcomes, but, interestingly, this particular T cell population has been implicated as being protective of autoimmune destruction of beta cells in type 1 diabetes [39].
5. Conclusion
In summary, we found that VSG in HFS fed mice altered the immune cell populations residing in the eWAT in a weight-independent manner. The CD11c− ATM population increased in number causing an altered ratio of the CD11c+/CD11c− ATMs in eWAT. The T cell populations (CD4+, CD8+, and double negative T cells) all expanded 3-fold and there was a significant drop in the proportion of ATDCs. Also we found that the FFA response to feeding and fasting was significantly enhanced with surgery and that VSG induced a significant upregulation of cytokine levels in plasma. These strong weight-independent changes in immune populations after surgery suggests that the immune system could play a significant role in the weight-independent adipose metabolic improvements observed after VSG in rodents.
Author contributions
HFS carried out the animal work, collected tissue, analyzed the data, and wrote the manuscript; BFZ collected tissue and set up the flow cytometry analysis; BFZ, RWO, DAS, CNL, and RJS all contributed to the discussion and reviewed and edited the manuscript; RJS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
The authors thank Tianna Vandermisen (University of Michigan) for her assistance with the animals. Also we thank the surgeons carrying out the VSG procedure: Alfor Lewis, Andriy Myronovych, and Mouhamadoul Toure (all University of Michigan).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.02.004.
Duality of interest
This study was funded by NIH grants R01DK097449 (RWO), R01DK090262 (CNL), R01 DK093848 (HFS and RJS); DK082480 (DAS); T32 AI007413-19 (BFZ); American Diabetes Association grant 07-12-CD-08 (BFZ), and NIDDK F31 DK103524 (BFZ). HFS, BFZ, RWO, and CNL all declare no conflict of interest. RJS receives financial support from Novo Nordisk, Boerhinger Ingelheim, Sanofi, and Ethicon Endo-Surgery. He has also served as a paid consultant for Novo Nordisk, Boehringer Ingelheim, Ethicon Endo-Surgery, Sanofi, Novartis, Circuit Therapeutics, Nestle, Daichii Sankyo, and Takeda. DS has received research support from Novo Nordisk, Ethicon Endo Surgery, and Boehringer Ingelheim. DS has also served as paid speaker for Novo Nordisk.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Gulliford M.C., Charlton J., Booth H.P., Fildes A., Khan O., Reddy M., editors. Costs and outcomes of increasing access to bariatric surgery for obesity: cohort study and cost-effectiveness analysis using electronic health records. 2016. Southampton (UK) [PubMed] [Google Scholar]
- 2.Frikke-Schmidt H., Seeley R.J. Defending a new hypothesis of how bariatric surgery works. Obesity (Silver Spring) 2016;24(3):555. doi: 10.1002/oby.21444. [DOI] [PubMed] [Google Scholar]
- 3.Arble D.M., Sandoval D.A., Seeley R.J. Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia. 2015;58(2):211–220. doi: 10.1007/s00125-014-3433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeley R.J., Chambers A.P., Sandoval D.A. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metabolism. 2015;21(3):369–378. doi: 10.1016/j.cmet.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen M., Lund M.T., Gregers E., Kraunsoe R., Van Hall G., Helge J.W. Adipose tissue mitochondrial respiration and lipolysis before and after a weight loss by diet and RYGB. Obesity (Silver Spring) 2015;23(10):2022–2029. doi: 10.1002/oby.21223. [DOI] [PubMed] [Google Scholar]
- 6.Lofgren P., Andersson I., Adolfsson B., Leijonhufvud B.M., Hertel K., Hoffstedt J. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. The Journal of Clinical Endocrinology and Metabolism. 2005;90(11):6207–6213. doi: 10.1210/jc.2005-0596. [DOI] [PubMed] [Google Scholar]
- 7.Nelson D., Porta R., Blair K., Carter P., Martin M. The duodenal switch for morbid obesity: modification of cardiovascular risk markers compared with standard bariatric surgeries. The American Journal of Surgery. 2012;203(5):603–608. doi: 10.1016/j.amjsurg.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Oberbach A., Schlichting N., Neuhaus J., Kullnick Y., Lehmann S., Heinrich M. Establishing of a reliable multiple reaction monitoring-based method for the quantification of obesity associated comorbidities in serum and adipose tissue requires intensive clinical validation. Journal of Proteome Research. 2014;13(12):5784–5800. doi: 10.1021/pr500722k. [DOI] [PubMed] [Google Scholar]
- 9.Grant R., Youm Y.H., Ravussin A., Dixit V.D. Quantification of adipose tissue leukocytosis in obesity. Methods in Molecular Biology. 2013;1040:195–209. doi: 10.1007/978-1-62703-523-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. Journal of Clinical Investigation. 2011;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiely A., McClenaghan N.H., Flatt P.R., Newsholme P. Pro-inflammatory cytokines increase glucose, alanine and triacylglycerol utilization but inhibit insulin secretion in a clonal pancreatic beta-cell line. Journal of Endocrinology. 2007;195(1):113–123. doi: 10.1677/JOE-07-0306. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante A.W., Jr. The immune cells in adipose tissue. Diabetes, Obesity and Metabolism. 2013;15(Suppl. 3):34–38. doi: 10.1111/dom.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X., Grijalva A., Skowronski A., van Eijk M., Serlie M.J., Ferrante A.W., Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metabolism. 2013;18(6):816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho K.W., Zamarron B.F., Muir L.A., Singer K., Porsche C.E., DelProposto J.B. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. Journal of Immunology. 2016;197(9):3650–3661. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature Medicine. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 16.Cho K.W., Morris D.L., DelProposto J.L., Geletka L., Zamarron B., Martinez-Santibanez G. An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Reports. 2014;9(2):605–617. doi: 10.1016/j.celrep.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrante A.W., Jr. Macrophages, fat, and the emergence of immunometabolism. Journal of Clinical Investigation. 2013;123(12):4992–4993. doi: 10.1172/JCI73658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou A.P., Paziuk M., Luevano J.M., Jr., Machineni S., Turnbaugh P.J., Kaplan L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Science Translational Medicine. 2013;5(178):178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahlstrom A., Sayin S.I., Marschall H.U., Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabolism. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Wilson-Perez H.E., Chambers A.P., Ryan K.K., Li B., Sandoval D.A., Stoffers D. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–2385. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Santibanez G., Lumeng C.N. Macrophages and the regulation of adipose tissue remodeling. Annual Review of Nutrition. 2014;34:57–76. doi: 10.1146/annurev-nutr-071812-161113. [DOI] [PubMed] [Google Scholar]
- 22.Kosteli A., Sugaru E., Haemmerle G., Martin J.F., Lei J., Zechner R. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. Journal of Clinical Investigation. 2010;120(10):3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomie C., Blasco-Baque V., Klopp P., Nicolas S., Waget A., Loubieres P. Triggering the adaptive immune system with commensal gut bacteria protects against insulin resistance and dysglycemia. Molecular Metabolism. 2016;5(6):392–403. doi: 10.1016/j.molmet.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tremaroli V., Karlsson F., Werling M., Stahlman M., Kovatcheva-Datchary P., Olbers T. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metabolism. 2015;22(2):228–238. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlotnikov-Klionsky Y., Nathansohn-Levi B., Shezen E., Rosen C., Kagan S., Bar-On L. Perforin-positive dendritic cells exhibit an immuno-regulatory role in metabolic syndrome and autoimmunity. Immunity. 2015;43(4):776–787. doi: 10.1016/j.immuni.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Magalhaes I., Pingris K., Poitou C., Bessoles S., Venteclef N., Kiaf B. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. Journal of Clinical Investigation. 2015;125(4):1752–1762. doi: 10.1172/JCI78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan F.H., Kohli R. Bariatric surgery: the rise and fall of bile acids. Surgery for Obesity and Related Diseases. 2016;12(4):770–771. doi: 10.1016/j.soard.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Haselow K., Bode J.G., Wammers M., Ehlting C., Keitel V., Kleinebrecht L. Bile acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and reduce proinflammatory capability of human macrophages. Journal of Leukocyte Biology. 2013;94(6):1253–1264. doi: 10.1189/jlb.0812396. [DOI] [PubMed] [Google Scholar]
- 30.Pressler J.W., Haller A., Sorrell J., Wang F., Seeley R.J., Tso P. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes. 2015;64(2):498–507. doi: 10.2337/db14-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mul J.D., Begg D.P., Haller A.M., Pressler J.W., Sorrell J., Woods S.C. MGAT2 deficiency and vertical sleeve gastrectomy have independent metabolic effects in the mouse. American Journal of Physiology – Endocrinology and Metabolism. 2014;307(11):E1065–E1072. doi: 10.1152/ajpendo.00376.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arble D.M., Sandoval D.A., Turek F.W., Woods S.C., Seeley R.J. Metabolic effects of bariatric surgery in mouse models of circadian disruption. International Journal of Obesity (London) 2015;39(8):1310–1318. doi: 10.1038/ijo.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanton M.C., Chen S.C., Jackson J.V., Rojas-Triana A., Kinsley D., Cui L. Inflammatory signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. Journal of Inflammation (London) 2011;8:8. doi: 10.1186/1476-9255-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Y., Yu S., Lambert J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. European Journal of Nutrition. 2014;53(1):149–158. doi: 10.1007/s00394-013-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Heijden R.A., Sheedfar F., Morrison M.C., Hommelberg P.P., Kor D., Kloosterhuis N.J. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging (Albany NY) 2015;7(4):256–268. doi: 10.18632/aging.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dror E., Dalmas E., Meier D.T., Wueest S., Thevenet J., Thienel C. Postprandial macrophage-derived IL-1 beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nature Immunology. 2017;18(3):283–292. doi: 10.1038/ni.3659. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obesity Reviews. 2010;11(1):11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 38.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J. Normalization of obesity-associated insulin resistance through immunotherapy. Nature Medicine. 2009;15(8):921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford M.S., Chen W., Wong S., Li C., Vanama R., Elford A.R. Peptide-activated double-negative T cells can prevent autoimmune type-1 diabetes development. European Journal of Immunology. 2007;37(8):2234–2241. doi: 10.1002/eji.200636991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.