Abstract
Purpose:
To compare the safety, efficacy, and clinical outcomes of simple limbal epithelial transplantation (SLET) with conjunctival-limbal autologous transplantation (CLAU) in severe unilateral ocular chemical burns.
Materials and Methods:
Twenty patients of unilateral chronic ocular burns with more than 270° limbal stem cell deficiency and a healthy fellow eye were divided into two groups – ten patients of Group A underwent SLET while ten patients of Group B were operated for CLAU. Patients were followed up for 6 months and assessed for a stable epithelialized ocular surface, extent of reduction in vascularization and forniceal reconstruction, improvement in corneal clarity and visual acuity.
Results:
A stable epithelialized corneal surface was obtained in all patients, with a significant reduction in the mean clock hours of vascularization in both the groups (P < 0.001). The mean symblepharon score showed a statistically significant reduction from 1.80 ± 1.14 to 0.30 ± 0.63 in Group A and 1.70 ± 1.06 to 0.15 ± 0.24 in Group B at 6 months. Corneal clarity, as well as best-corrected visual acuity, showed a statistically significant improvement in both the groups.
Conclusion:
Both the procedures, SLET and CLAU, were equally effective in achieving a stable ocular surface, forniceal reconstruction, and regression of corneal vascularization. The requirement of minimal donor tissue in SLET makes it a preferred option over CLAU in cases of uniocular chronic ocular burns.
Keywords: Chemical burns, conjunctival-limbal autologous transplantation, limbal stem cell deficiency, simple limbal epithelial transplantation
Ocular burns represent 7%–18% of the eye injuries, and a vast majority of them have a chemical etiology.[1] Ocular surface disease resulting from chemical burns has been demonstrated to be a manifestation of limbal dysfunction. Limbal stem cell destruction leads to conjunctivalization, neovascularization, chronic inflammation, and persistent epithelial defects on the cornea.[2,3] In addition, chemical burns induce damage to the conjunctival epithelium and goblet cells, which results in severe dry eye with keratinization, symblepharon formation, and scarring of eyelids.
All the management options for chronic chemical injuries aim at restoration of a healthy ocular surface by transplantation of limbal stem cells with or without amniotic membrane transplantation for symblepharon release.[4,5,6]
Kenyon and Tseng recommended conjunctival transplantation including limbal epithelium (CLAU) (3–6 clock hours) from the healthy fellow eye to be a successful approach for the treatment of widespread ocular surface damage induced due to limbal stem cell deficiency (LSCD) from chemical or thermal burns.[4] Later, many studies[2,7] have elaborated on successful results when limbal grafts have been used along with amniotic membrane to restore ocular surface. Although the risk of donor site developing LSCD[8] exists, it is rare as long as <6 clock hours of limbus is harvested.[9]
To avoid complication at donor site due to limbal harvesting, cultivated limbal epithelial transplantation (CLET)[5] has been suggested as an alternative procedure and has been successfully practiced worldwide.[6,10,11] Maintenance of an elaborate laboratory for ex vivo cultivation of limbal stem cells adds to the expenses and is not possible for every set-up. Sangwan et al.[12] described simple limbal epithelial transplantation (SLET), a novel surgical technique to restore ocular surface stability in severe LSCD in a pilot study of six patients. SLET combines the benefits of CLAU and CLET and avoids the difficulties of either.
Later, autologous SLET has been documented to be an effective and safe modality for the treatment of unilateral LSCD in many case reports and studies.[13,14,15,16,17,18,19] Clinical success rates and visual acuity improvement have been reported to be equal to or better than those reported with earlier techniques.[13]
To the best of our knowledge, there has been no prospective randomized study, comparing the results of CLAU with SLET for the management of severe unilateral ocular burns. Therefore, a study was designed in 2013 in an attempt to compare the safety and efficacy of standard procedure (i.e., CLAU) with a pilot procedure (i.e., SLET) in unilateral chronic chemical burns with more than 270° LSCD.
Materials and Methods
Study design
A prospective study was conducted at a tertiary eye center from January 2014 to March 2015, following the ethical clearance from the Ethical Committee of the Institute. Patients of unilateral chronic ocular burns (defined as no history or clinical signs of ocular surface disease in the fellow eye) of more than 3 months duration, more than 270° LSCD, and no prior history of limbal transplantation were included in the study. LSCD was defined as total or partial superficial corneal vascularization, punctate corneal surface staining on fluorescein, conjunctivalization of the corneal surface, and absence of limbal palisades of Vogt. Only patients above 2 years of age were included in the study. The study followed the tenets of Declaration of Helsinki. A written, informed consent was obtained from the patients of >18 years of age and of legal guardian of patients of <18 years. Assent from children of >13 years was also taken. Patients of acute ocular burn, prior history of limbal transplant, severe dry eye, entropion, ectropion, lagophthalmos, glaucoma, and infection were excluded from the study. The follow-up was done with patients at regular intervals and outcome parameters were assessed and analyzed at 6 months.
This study has been taken as an academic exercise which was completed within 15 months. On average, incidence of chronic chemical ocular burns is around 25–30/year at our institution. Therefore, the possibility to carry out the study in large number of cases within a fixed period was not feasible.
In view of the above, this pilot study was undertaken with twenty cases of chronic chemical burns with more than 270° LSCD, satisfying the inclusion and exclusion criteria. They were randomly divided into two groups, of ten cases each, using computer-generated tables. Group A underwent SLET and Group B underwent CLAU.
Outcome measures of efficacy
The primary outcome measures included ocular surface stability in terms of epithelialized stable corneal surface, extent of reduction in vascularization (clock hours), forniceal reconstruction of the recipient eye, following SLET or CLAU at 6 months. Secondary outcome measures were improvement in corneal clarity and visual acuity.
Outcome measure of safety
The observation of intraoperative and postoperative complications of SLET/CLAU in the recipient eye and limbal biopsy sites in the donor eye was taken as safety outcome measures.
Surgical technique
Adult patients were operated under peribulbar anesthesia, while children under 16 years were administered general anesthesia. All surgeries were performed by a single surgeon.
Simple limbal epithelial transplantation
Before the procedure, a limbal lenticule of 2 mm × 2 mm with adjacent conjunctiva was harvested from the healthy fellow donor eye and placed in balanced salt solution. A 360° peritomy was done in the recipient eye and the vascular pannus covering the cornea was removed with symblepharon release and removal of sub-conjunctival fibrotic tissue was performed. Sub-conjunctival dissection was done up to 12–14 mm from the limbus after isolating the recti. After cauterization of bleeding points, thawed cryopreserved human amniotic membrane (hAM) was spread over the bare ocular surface and was tucked under the conjunctival edges with epithelial side up. Clean, processed hAM wrapped onto a sheet of nitrocellulose paper (5 mm × 5 mm) stored in 1:1 mixture of glycerol and Dulbecco's modified Eagle medium at −70°C was used. The hAM was anchored to the recessed conjunctival edges with 8-0 vicryl sutures (Ethicon, Johnson and Johnson, Ahmedabad, India) with episcleral hitching and fibrin glue (TISSEEL Kit from Baxter AG, Vienna, Austria). The donor limbal lenticule harvested earlier was cut into 10–12 small pieces using Vannas scissors and placed on hAM, with the epithelial side up, in a circular fashion around the center of the cornea, avoiding visual axis. The transplants were fixed in place using fibrin glue. A soft bandage contact lens was then placed on both the donor and recipient eyes. Thereafter, appropriate-sized conformer or symblepharon ring was put and temporary tarsorrhaphy was done.
CLAU
Superficial vascularized scar tissue covering the cornea was removed from the injured eye after doing conjunctival peritomy, approximately 2 mm posterior to the limbus. The limbal area of the affected eye was exposed, and host bed was prepared. The conjunctiva was recessed as far as possible, symblepharon was released, and sub-conjunctival fibrous tissue was removed, after hooking the recti and achieving good exposure. The bleeders were cauterized. Two limbal lenticules each of 6–8 mm width with 2 mm bulbar conjunctiva from 10.30 to 1.30 o’clock position and 4.30 to 7.30 o’clock position were harvested from the donor eye, using microscissors. These conjunctival limbal grafts, each of 3 clock hours, were transferred preferentially to the 12 and 6 o’clock positions of the affected eye and secured in their anatomical position, using fibrin glue. The hAM was put over the cornea and anchored to the recessed conjunctival margins with 8-0 vicryl suture and episcleral hitching and fibrin glue. A soft bandage contact lens was then placed on both the donor and recipient eyes. After putting an appropriate-sized conformer or symblepharon ring, a temporary tarsorrhaphy was done. Both the eyes were patched overnight after instilling two drops of topical 0.3% moxifloxacin (Cipla India, Mumbai, India) in each eye.
Postoperatively, in both the procedures, patients were prescribed tobramycin 0.3% (Tobrex, Alcon Laboratories Pvt. Ltd., Bengaluru, India) and carboxymethyl cellulose 0.5% eye drops (Allergan India Pvt. Ltd., Bengaluru, India) four times a day (in both eyes) and loteprednol 1% (Sun Pharmaceuticals Pvt. Ltd, Mumbai, India) eye drops four times a day (operated eye only), tapered weekly over 6 weeks. Follow-ups were conducted every day till day 7, then weekly till 4 weeks after surgery. The bandage contact lens was removed from the donor eye on day 7. Symblepharon ring or conformer was removed with release of tarsorrhaphy at 4 weeks. Thereafter, the patients were followed at 2 weeks intervals till 6 months.
Statistical analysis
Statistical analysis of the data was done using unpaired t-test/Mann–Whitney U-test for quantitative variables and Chi-square test for the qualitative variables in the two groups. Unpaired t-test/Wilcoxon test was used for the comparison between postoperative and preoperative variables within the group using the SPSS version 17 (Statistical Package for the Social Sciences, IBM, NY, United States).
Results
The demographic profile, preoperative details, and type of procedure for each patient are depicted in Table 1.
Table 1.
The preoperative status with regard to cause of injury, duration, epithelial defect, severity of symblepharon, corneal vascularization, visual acuity, grade of corneal clarity, and procedure performed
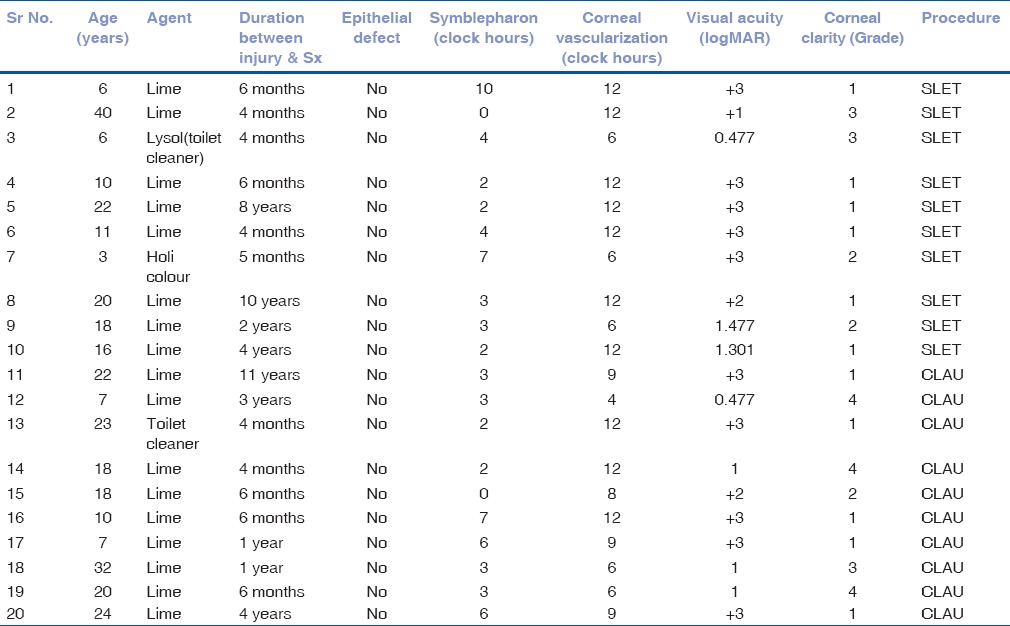
The age of the patients in the study ranged from 3 to 40 years. The mean age of patients in Group A was 15.20 ± 10.83 years while in Group B was 18.10 ± 8.05 years. The mean duration between injury and surgery was 22.9 months in Group A and 26.5 months in Group B, the range being 4 months–11 years. None of the patients had any prior ocular surgery. The causative agents for the chemical burn in the study were lime (17), toilet cleaner (2), and dry Holi festival color (1). There were 13 male and 7 female patients in the study.
Corneal epithelial defect
There was no corneal epithelial defect in either group preoperatively or postoperatively.
Corneal vascularization
Corneal vascularization was graded from 0 to 12 clock hours. The mean clock hour vascularization in Group A decreased significantly from 10.20 ± 2.90 clock hours preoperatively to 3.70 ± 2.67 and 2.10 ± 2.60 clock hours postoperatively at 3 and 6 months, respectively. Mean vascularization in Group B reduced significantly from 8.70 ± 2.79 clock hours preoperatively to 2.50 ± 1.08 and 2.50 ± 2.42 clock hours postoperatively at 3 and 6 months, respectively. However, there was no statistically significant difference in vascularization between the two groups at 3 and 6 months [Fig. 1].
Figure 1.
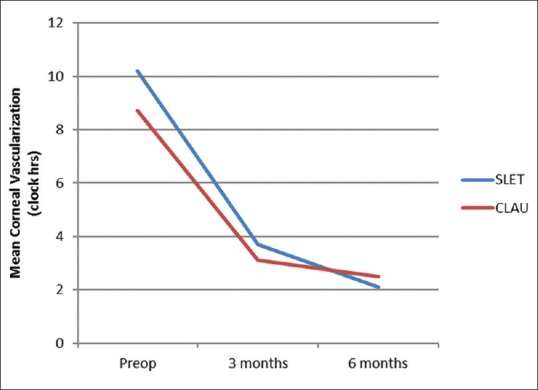
Decrease in mean clock hours of corneal vascularization from preoperative status (10.2/8.7 in Groups A and B) to postoperative status (2.10/2.42 in Groups A and B), respectively
Symblepharon
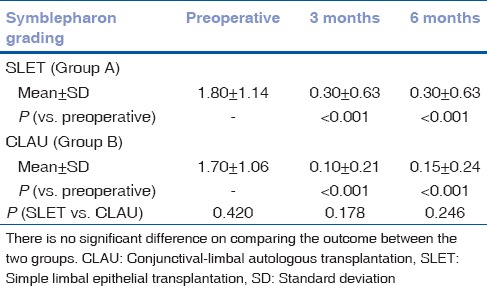
Symblepharon resulting in forniceal obliteration due to scar tissue formation was graded from 0 to 4. Score of 1 was given for every 3-clock hour involvement. The mean symblepharon score preoperatively was 1.80 ± 1.14 in Group A and 1.70 ± 1.06 in Group B. There was a reduction in this score from preoperative value to 0.30 ± 0.63 at 3 months and this did not change at 6 months in Group A. This score was 0.10 ± 0.2 and 0.15 ± 0.24 in Group B at 3 and 6 months, respectively. The reduction in the symblepharon score was statistically significant from preoperative status in both the groups at 3 and 6 months. However, there was no statistically significant difference in the symblepharon status between the two groups at 3 and 6 months [Fig. 2 and Table 2].
Figure 2.
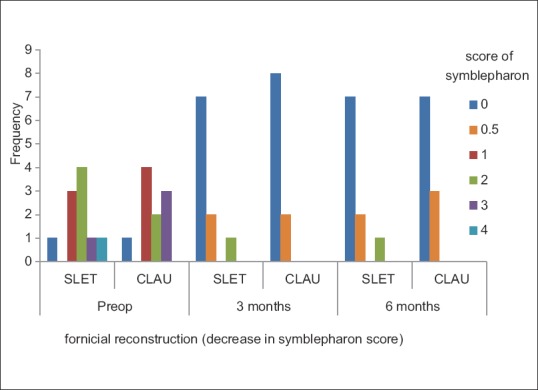
Significant reduction in score of symblepharon following forniceal reconstruction in both the groups
Table 2.
A statistically significant reduction in symblepharon score following simple limbal epithelial transplantation and conjunctival-limbal autologous transplantation (P<0.001)
Corneal clarity
Corneal clarity was graded from 1 to 4 based on the assessment of iris and pupil on slit lamp examination, with 1 being opaque cornea and no view of underlying iris or pupil and 4 being clear cornea. The mean corneal clarity in Group A was 1.60 ± 0.84, which improved significantly to 2.50 ± 0.53 and 3.10 ± 0.57 at 3 and 6 months, respectively.
The mean corneal clarity in Group B was 2.20 ± 1.40, which improved significantly to 2.80 ± 0.92 and 3.00 ± 0.82 at 3 and 6 months, respectively. However, there was no statistically significant difference in clarity between the two groups [Fig. 3a–d].
Figure 3.
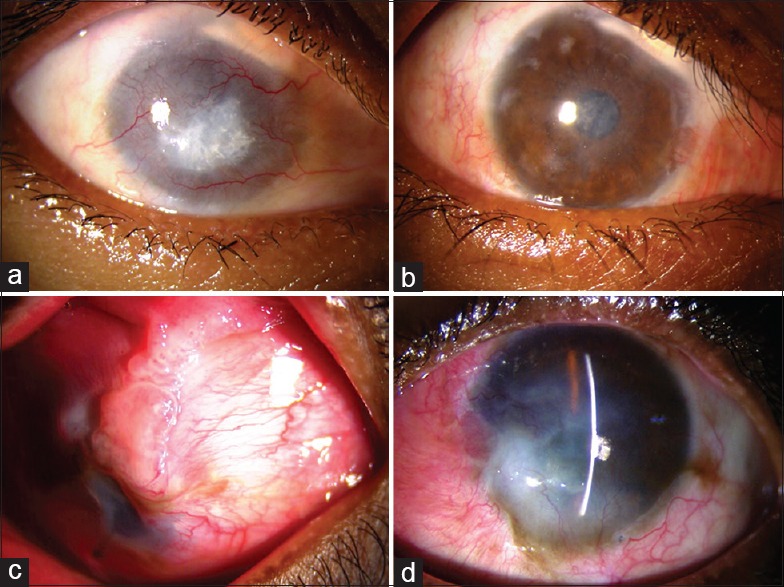
(a) Eye showing 360° limbal stem cell deficiency, corneal vascularization and superior symblepharon from 12 to 2 o’clock with retained calcium particles. (b) Same eye 6 months postsimple limbal epithelial transplantation (Group A) – an avascular, epithelialized corneal surface, improved corneal clarity and corrected symblepharon. Few remnants of limbal transplant are visible on the surface. (c) Eye showing superior symblepharon with exuberant granulation tissue from 12 to 3 o'clock covering more than half of cornea. (d) Six months post-CLAU (Group B) epithelialized corneal surface, improved corneal clarity, regressed vascularization, and corrected symblepharon
Visual acuity
In both groups, visual acuity ranged from perception of light to 6/18 preoperatively and postoperatively. For the purpose of statistical analysis, perception of light and hand movements were taken as +3 in logarithm of minimum angle of resolution (logMAR) and finger counting at 2 m as +2 in logMAR.[20] 6/6 was graded as 0 in logMAR.
The mean preoperative logMAR best-corrected visual acuity (BCVA) in Group A was 2.13 ± 1.0, which improved to 1.53 ± 0.72 and 1.62 ± 0.86 at 3 and 6 months, respectively. The mean preoperative logMAR BCVA in Group B was 2.05 ± 1.07, which improved to 1.45 ± 0.75 and to 1.23 ± 0.57 at 3 and 6 months, respectively. The evaluation of BCVA was possible with spectacles in eight patients only. This postoperative improvement in vision in both the groups was statistically significant over the preoperative vision. There was no significant difference in the postoperative vision at 6 months between the two groups.
Donor eye
In both the groups, donor site/sites reepithelialized and were covered with conjunctival epithelium. None of the donor sites demonstrated any donor site LSCD. There were mild congestion and chemosis for only 3–4 days postoperatively at the donor site/sites. The average healing time at the donor site/sites was 5 ± 2 days in Group A and 8 ± 2 days in Group B.
Visual acuity in donor eye
Visual acuity was recorded in adults using the Snellen chart, while in children, the assessment was made using the Snellen picture chart or Kay's picture chart. The BCVA ranged from 6/12 to 6/6 (0.30–0 in logMAR) in Group A and 6/9 to 6/6 (0.18–0 in logMAR) in Group B, both preoperatively and postoperatively at 3 and 6 months. There was no change from the preoperative status in the visual acuity in the donor eye, in both the groups at 3 and 6 months postoperatively.
Complications
No major complication was seen in any of the cases, except Case No. 14, which developed thinning and a micro-perforation at 3 months, which healed with patching leading to corneal opacity. Hemorrhage under the amniotic membrane was seen in one case (Case No. 2), which resolved spontaneously. No complication was observed at donor site.
Discussion
CLAU was reported to provide successful cure of widespread LSCD first by Kenyon and Tseng.[4] They recommended conjunctival transplantation along with limbal epithelium for ocular surface reconstruction in cases of extensive conjunctival inflammation, scarring, or loss of limbal and conjunctival epithelium. Near complete surgical success with supplemental amniotic membrane transplantation and CLAU in cases of ocular burns has also been reported in many studies.[2,3] hAM plays a critical role in promoting and preserving the ability of the limbal epithelial stem cells to proliferate.[21,22]
Pellegrini et al.[5] later suggested CLET as an alternative procedure, wherein <1 clock hour of donor limbus could be expanded ex vivo into transplantable epithelial sheet. The success rate and visual acuity outcome of CLET have been found to be comparable between autografts and allografts.[10] The need for an expensive laboratory set-up requiring various regulatory guidelines is the major drawback of CLET, restricting its access to limited centers.
Long-term changes at the donor sites and safety implications for donor eyes used for harvesting tissue for autologous transplant have been studied, and no significant donor site complications have been reported.[23] The technique of SLET introduced by Sangwan et al.[12] in 2012 proved to be successful in maintaining ocular surface stability in severe LSCD in six patients in the pilot study. In this technique, <1 clock hour of donor limbus is harvested from the healthy fellow eye. The advantage of the technique is that it requires less donor tissue than that used for conventional autografting and elimination of the need of a specialist laboratory for cell expansion. Further results of success of SLET in effectively treating unilateral LSCD following ocular burns have also been reported in recent times.[13,14,15,16,17,18,19] Results from a multicenter retrospective study have described nearly 83% success rate of SLET, suggesting it to be comparable to, or even better than, previous techniques. There is, however, no comparison of the study results with other techniques and only historical data have been used. There does not exist a prospective study, comparing different techniques of limbal transplantation in severe unilateral ocular chemical burns performed in a similar clinical setting.
We conducted a prospective study on twenty patients with chronic ocular burns, with more than 270° LSCD who underwent either SLET or CLAU from the healthy fellow eye. The outcomes of the two procedures were compared with regard to ocular surface reconstruction, improvement in corneal clarity and visual acuity at 6 months.
Ocular surface reconstruction with reference to symblepharon and forniceal reconstruction was achieved in all the cases. Limbal transplantation in the form of CLAU/SLET was equally successful in the correction of symblepharon in 66.6% of cases with the formation of deep fornices in each group. There was statistically no significant difference in the symblepharon scoring at 6 months postoperatively between the two groups. Partial success was achieved in the remaining 33.33% of the patients as there was recurrence of symblepharon at 6 months. This recurrence, however, was much less than the preoperative intensity. The presence of symblepharon preoperatively has been reported to be a risk factor for failure of SLET.[13,19] The epithelial stem cells from the limbus, presumably serve as a barrier to conjunctivalization of the cornea and thus are successful in correcting symblepharon. Meallet et al.[24] and Kheirkhah et al.[25] showed success in symblepharon lysis and formation of deep fornix in three patients of LSCD who underwent CLAU. Sangwan et al. reported 100% success in three patients of LSCD with symblepharon, who underwent SLET during a mean follow-up of 9.2 ± 1.9 months.[12] However, there is no mention of symblepharon results in the report of SLET in larger series.[19] Complete removal of the scar tissue, deeper placement of the amniotic membrane with episcleral hitching, and additional limbal transplant in the present study aided in equal and effective forniceal reconstruction with both techniques of limbal transplantation achieving comparable results.
The corneal clarity improved in both the groups from the preoperative status. However, the difference in the mean corneal clarity preoperatively and at 3 and 6 months postoperatively between the two groups was not statistically significant (P = 0.130). Improvement in corneal clarity was due to epithelial clarity and the limbal transplant induced underlying stromal remodeling. Meallet et al.[24] and Rama et al.[26] showed improvement in corneal clarity in patients of LSCD, who underwent limbal stem cell transplant.
In our study, both Groups A and B showed a significant decrease in vascularization at 3 months from preoperative status, with further improvement at 6 months in Group A. The decrease in vascularization between the two groups at 3 and 6 months postoperatively was not statistically significant. Kenyon and Tseng reported a decrease in neovascularization in 9 patients and regression of neovascularization in 6 cases out of 22 patients with LSCD who underwent CLAU during a mean follow-up of 18 months.[4] Basu et al. have reported a successful outcome over a long-term in 76% of the cases undergoing SLET.[19] Equally promising outcomes have been reported in multicenter studies with regard to regeneration of an avascular, healthy corneal surface.[13]
This regression of neovascularization and the arrest of new blood vessel formation following SLET/CLAU are probably due to the inhibitory effect of corneal epithelium on ocular surface neovascularization. An avascular bed also aids in the process of visual rehabilitation with later penetrating/lamellar keratoplasty.
In both the groups, the postoperative improvement in vision was statistically significant over the preoperative vision. Visual acuity improved statistically significantly from preoperative status to 3 months and further with little change at 6 months. There was no significant difference in the postoperative vision at 6 months between the two groups. Improvement in visual acuity could be attributed to improved corneal epithelial clarity, remodeling of underlying stroma, and decreased vascularization. Studies[4,5,7,12,17,19] using varying techniques of limbal stem cell transplantation have also shown significant improvement in visual acuity.
Our study found comparable outcomes for both, SLET and CLAU with regard to ocular surface and forniceal reconstruction, regression of corneal vascularization, improvement in corneal clarity, and visual acuity in cases with severe LSCD. Both the techniques were found to be safe for the donor eye. As SLET offers the advantage of requirement of smaller amount of donor tissue, SLET should be preferred in cases of unilateral chronic ocular burns over CLAU. In the event of failure of SLET, there remains the possibility of safely harvesting further limbus for a repeat surgery. Prior management with these forms of limbal stem cell transplantation helps in the success of visual rehabilitation measures performed later. This small first-time comparison with 6-month follow-up between CLAU and SLET in the management of chronic chemical burns with severe LSCD may serve as a basis of further long-term trials with more patients.
Short-term follow-up of 6 months is the weakness of the study. Therefore, similar study with more number of cases and a longer follow-up is recommended for conclusive results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We all have contributed significantly to the collection and interpretation of data, preparation, and final approval of the manuscript.
References
- 1.Merle H, Gérard M, Schrage N. Ocular burns. J Fr Ophthalmol. 2008;31:723–34. doi: 10.1016/s0181-5512(08)74391-2. [DOI] [PubMed] [Google Scholar]
- 2.Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–76. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- 3.Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–41. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 6.Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br J Ophthalmol. 2011;95:1525–9. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 7.Gomes JA, dos Santos MS, Cunha MC, Mascaro VL, Barros Jde N, de Sousa LB. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110:466–73. doi: 10.1016/s0161-6420(02)01888-2. [DOI] [PubMed] [Google Scholar]
- 8.Basti S, Mathur U. Unusual intermediate-term outcome in three cases of limbal autograft transplantation. Ophthalmology. 1999;106:958–63. doi: 10.1016/S0161-6420(99)00516-3. [DOI] [PubMed] [Google Scholar]
- 9.Holland EJ, Schwartz GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996;15:549–56. [PubMed] [Google Scholar]
- 10.Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015;34:592–600. doi: 10.1097/ICO.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 11.Pauklin M, Fuchsluger TA, Westekemper H, Steuhl KP, Meller D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57–70. doi: 10.1159/000315020. [DOI] [PubMed] [Google Scholar]
- 12.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–4. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 13.Vazirani J, Ali MH, Sharma N, Gupta N, Mittal V, Atallah M, et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: Multicentre results. Br J Ophthalmol. 2016;100:1416–20. doi: 10.1136/bjophthalmol-2015-307348. [DOI] [PubMed] [Google Scholar]
- 14.Mittal V, Jain R, Mittal R, Vashist U, Narang P. Successful management of severe unilateral chemical burns in children using simple limbal epithelial transplantation (SLET) Br J Ophthalmol. 2016;100:1102–8. doi: 10.1136/bjophthalmol-2015-307179. [DOI] [PubMed] [Google Scholar]
- 15.Das S, Basu S, Sangwan V. Molten metal ocular burn: Long-term outcome using simple limbal epithelial transplantation. BMJ Case Rep 2015. 2015 doi: 10.1136/bcr-2014-209272. pii: Bcr2014209272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal V, Jain R, Mittal R. Ocular surface epithelialization pattern after simple limbal epithelial transplantation: An in vivo observational study. Cornea. 2015;34:1227–32. doi: 10.1097/ICO.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 17.Vazirani J, Basu S, Sangwan V. Successful simple limbal epithelial transplantation (SLET) in lime injury-induced limbal stem cell deficiency with ocular surface granuloma. BMJ Case Rep 2013. 2013 doi: 10.1136/bcr-2013-009405. pii: Bcr2013009405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhalekar S, Basu S, Lal I, Sangwan VS. Successful autologous simple limbal epithelial transplantation (SLET) in previously failed paediatric limbal transplantation for ocular surface burns. BMJ Case Rep 2013. 2013 doi: 10.1136/bcr-2013-009888. pii: Bcr2013009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu S, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple limbal epithelial transplantation: Long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology. 2016;123:1000–10. doi: 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 20.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–91. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 21.Mariappan I, Kacham S, Purushotham J, Maddileti S, Siamwala J, Sangwan VS. Spatial distribution of niche and stem cells in ex vivo human limbal cultures. Stem Cells Transl Med. 2014;3:1331–41. doi: 10.5966/sctm.2014-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SY, Han B, Zhu YT, Mahabole M, Huang J, Beebe DC, et al. HC-HA/PTX3 purified from amniotic membrane promotes BMP signaling in limbal niche cells to maintain quiescence of limbal epithelial progenitor/stem cells. Stem Cells. 2015;33:3341–55. doi: 10.1002/stem.2091. [DOI] [PubMed] [Google Scholar]
- 23.Miri A, Said DG, Dua HS. Donor site complications in autolimbal and living-related allolimbal transplantation. Ophthalmology. 2011;118:1265–71. doi: 10.1016/j.ophtha.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Meallet MA, Espana EM, Grueterich M, Ti SE, Goto E, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003;110:1585–92. doi: 10.1016/S0161-6420(03)00503-7. [DOI] [PubMed] [Google Scholar]
- 25.Kheirkhah A, Blanco G, Casas V, Hayashida Y, Raju VK, Tseng SC. Surgical strategies for fornix reconstruction based on symblepharon severity. Am J Ophthalmol. 2008;146:266–75. doi: 10.1016/j.ajo.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–55. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]


