Figure 1. Therapeutic loading and release mechanisms from hydrogel-based delivery vehicles.
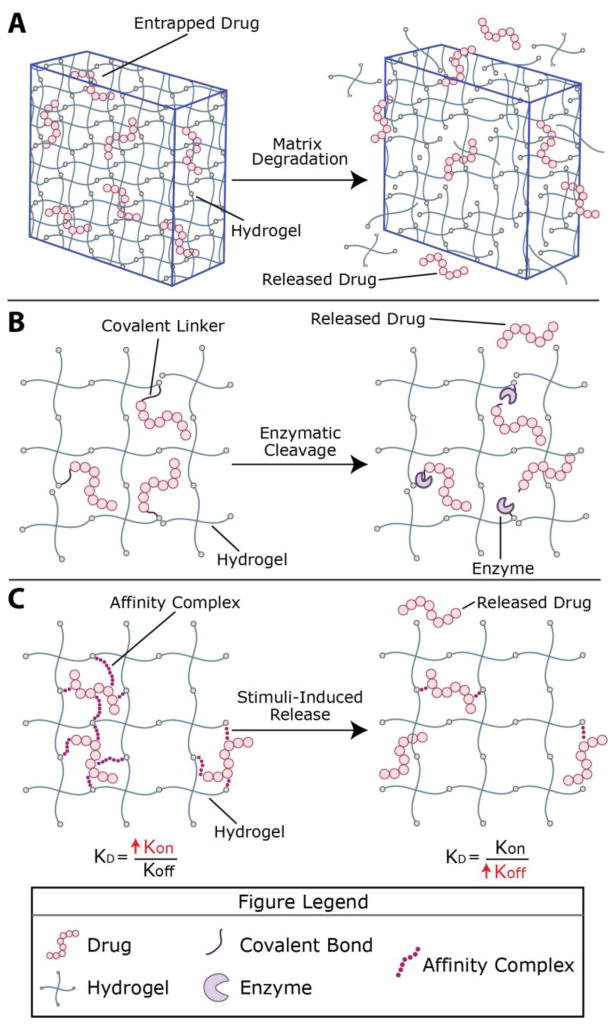
Hydrogels, including those formed by thiol–ene click chemistry, can be formed in vitro or in vivo for therapeutic delivery applications. Stoichiometric reaction between functional groups on multiarm poly(ethylene glycol) (PEG) macromers functionalized with specific alkenes or thiols, respectively, has been reported, producing thiol–ene hydrogels with nearly ideal network structure (shown here); these are of growing interest for delivery applications toward providing a well-defined and predictable mesh size. However, macromolecules of varied length and functionality, both of synthetic and natural origin and decorated with various alkenes or thiols, broadly have been utilized for the formation of thiol–ene hydrogels. Engineering of material structure and chemistry provide handles for controlling release profiles. A) For example, therapeutics frequently have been encapsulated within the hydrogel network, where the cargo is entrapped if the average pore size (e.g., mesh size) of the hydrogel is smaller than the drug; degradation of the network, which increases mesh size, controls release. B) Small molecule drugs or peptides, which can be difficult to entrap within hydrophilic highly-swollen hydrogels, have been tethered to the network and released upon tether cleavage (or complete network degradation); here, cleavage by a cell-secreted enzyme is depicted. C) Ligands for a therapeutic of interest also have been incorporated within hydrogels for controlling retention and release by affinity binding; reversible binding of the ligand dictated by kon/koff determines the fraction of bound/free therapeutic, where diffusion of the free species (or matrix degradation) controls release. These therapeutic loading and release mechanisms can be used in different combinations than those depicted here and have been used in thiol–ene hydrogels.
